Weliky Group Discovers Potential Basis for Chronic Infection by HIV
The Weliky Group in the MSU Chemistry Department has made the surprising and significant discovery that the fusion peptide region of the HIV Gp41 protein adopts a broad distribution of distinct structures, many of which are functional. This broad distribution may be an important basis for chronic infection by the virus.
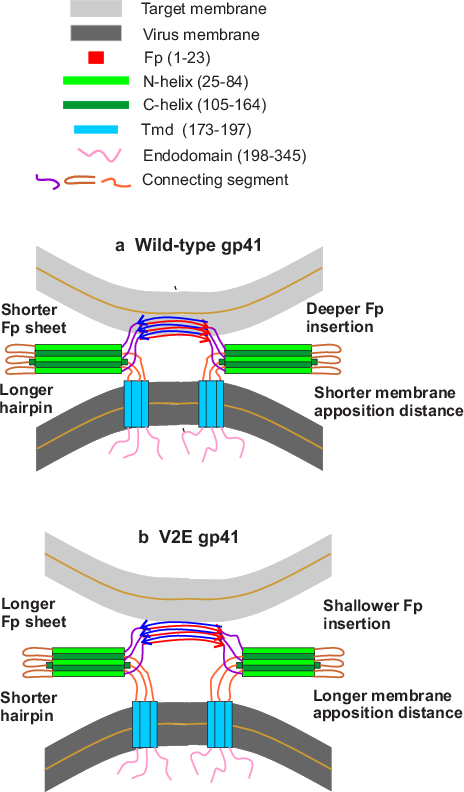
The peptide is required for joining (“fusion”) of HIV and cellular membranes, an early step of HIV infection. The fusion peptide is part of the spikes that protrude from the virus surface and is a target of the immune system, including antibodies. The fusion peptide is also an immunogenic component of a HIV vaccine in development.
The broad distribution of peptide structures may be an evolutionary solution by HIV to the competing requirements of maintaining fusion function while also undergoing continual mutation to evade the immune system. Many of the fusion peptide structures are functional, so some mutations simply shift populations between these different structures while also allowing escape from neutralization by the immune system.
The significance of the broad distribution is supported by additional Weliky group results on a peptide with the fusion-defective V2E mutation that results in non-infectious virus. There is also a broad structural distribution of the mutated peptide, but very different from that of the native functional peptide.

As part of this work, the Weliky group developed a new analysis approach to determine a single set of structural populations from nuclear magnetic resonance (NMR) data of many different samples.
“These findings and new method enable future exploration of how common these broad distributions are for pathogens that mutate to withstand sustained pressure from the immune system," David Weliky noted.
"This may be an optimal solution for processes that are coordinated movements of molecules, like membrane fusion.”
This new approach paves the way to discovering other proteins that also function using broad but defined distributions of structures. This work has been published in the Proceedings of the National Academy of Sciences



