Behind the 'black fungus' crisis: breakthrough research antifungal challenges
Mucormycosis, a dangerous fungal infection caused by Mucorales, poses a serious threat to individuals with weakened immune systems and diabetes.
For instance, during India’s 2021 COVID-19 surge, the infection became particularly severe, resulting in widespread cases of COVID-19-Associated Mucormycosis (CAM). At the time, these cases were casually referred to as "the black fungus," and presented significant diagnostic and therapeutic challenges.
The current first-line treatment for mucormycosis includes liposomal amphotericin B, supplemented by second-line agents such as azoles. Aggressive surgical removal of infected tissue is frequently required before initiating antifungal therapy.
However, Mucorales are insensitive to echinocandins, a newer class of antifungal drugs that target β-glucan synthesis in fungal cell walls, which has proven safe and effective against many other major fungal pathogens.
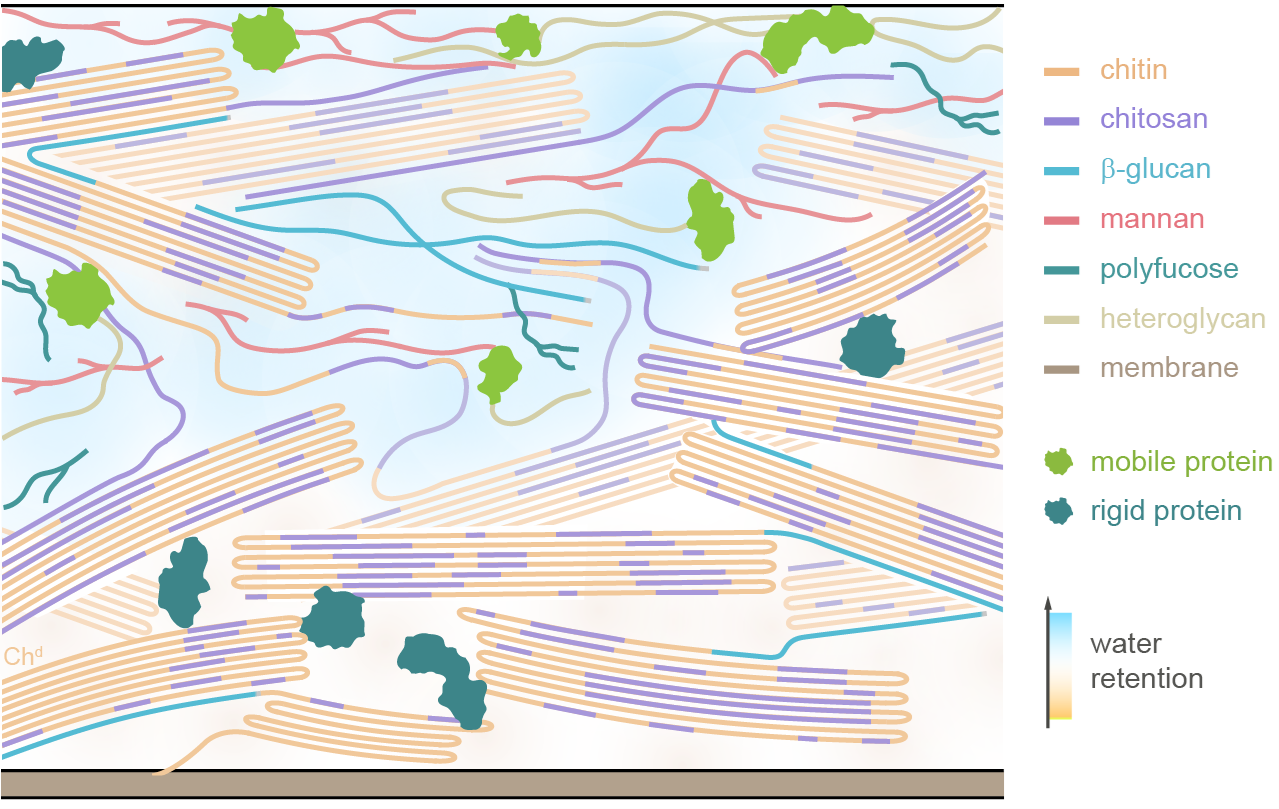
This inefficacy is believed to stem from the unique chitin-rich composition of Mucorales cell walls, whose molecular-level structure remains poorly understood. To address this knowledge gap, researchers from Michigan State University studied five key species of Rhizopus and Mucor, the primary pathogens behind mucormycosis and CAM, and evaluated the fungi's response to nikkomycin, the only available chitin inhibitor used in antifungal treatments. Their findings were published in Nature Communications.
“We discovered that Mucorales cell walls are primarily composed of crystalline yet highly variable chitin and chitosan, with small amounts of β-glucan binding exclusively to a specific form of this complex,” said Tuo Wang, lead author of the study and the Carl H. Brubaker Jr. Endowed Associate Professor in the Department of Chemistry at MSU’s College of Natural Science.

“Nikkomycin removes this β-glucan-chitin complex, which only accounts for a small portion of the cell wall, but does not compromise the overall cell wall structure, which explains why echinocandins are ineffective against these fungi,” Wang added.
These discoveries offer the first comprehensive understanding of the carbohydrate structures and nanoscale organization within Mucorales cell walls. They provide crucial insights into chitin biosynthesis and the function of chitin inhibitors, paving the way for the development of more targeted antifungal therapies designed to tackle the unique composition of Mucorales cell walls.
The study also emphasizes the necessity for a deeper understanding of the diverse families of chitin synthases and deacetylases, and other related synthetic pathways as potential targets for novel antifungal therapies
Wang’s team at MSU included postdoctoral researchers Dr. Qinghui Cheng and Dr. Jayasubba Reddy Yarava, as well as graduate students Malitha Dickwella Widanage and Ankur Ankur. The research also involved input from microbiologists Jean-Paul Latge at the University of Crete and Ping Wang from the LSU Health Sciences Center.
The team used solid-state Nuclear Magnetic Resonance (NMR) as a key methodology, allowing them to study living fungal cells without altering their chemical or physical properties. Data collection was supported by the Max T. Roger NMR facility at MSU and access to high-field magnets at the National High Magnetic Field Laboratory in Tallahassee, Florida.
“The solid-state NMR spectra of these samples, including those involving 13C, 15N, and 1H nuclei, are extraordinarily beautiful and unmatched by any other fungal species or whole-cell samples studied by our group to date,” noted Wang.