David P. Weliky

Research
Biophysical Chemistry and Nuclear Magnetic Resonance
(Research Description PDF)
We are a biophysical chemistry group that is focused on understanding the mechanism of entry by viruses enveloped by a membrane. Many important human pathogens are enveloped viruses, including Human Immunodeficiency (HIV), Influenza, Measles, Rabies, West Nile, Zika, Ebola, SARS, and MERS. Each virus has evolved a protein in its membrane that catalyzes the joining (“fusion”) of the virus membrane with the membrane of the target cell. We are studying the glycoprotein 41 kDa (gp41) fusion protein of HIV and hemagglutinin subunit 2 (HA2) fusion protein of influenza. Our work and contributions include the protein structures and locations in membrane. We also study the changes in membrane structure associated with the protein. A significant fraction of our effort is in development and application of “solid-state”, i.e. anisotropic nuclear magnetic resonance (NMR) to these proteins. We also apply a variety of other biophysical methods including circular dichroism spectroscopy, fluorescence spectroscopy, hydrogen-deuterium exchange mass spectrometry, X-ray crystallography, and electron microscopy. We also have a significant effort in protein synthesis and chromatographic purification that includes molecular biology and protein expression in bacteria, solid-phase peptide synthesis, and native chemical ligation. A side-project in the laboratory is NMR analysis of expressed proteins in bacterial inclusion bodies, which are commonly-formed solid protein aggregates. We want to understand why these aggregates form, and the degree of folding of individual proteins within the aggregates. Our NMR methodology focuses on quantitative determination of distributions of populations of protein structures and membrane locations, with a particular emphasis on the rotationalecho double-resonance (REDOR) approach which is robust and amenable to quantitative analysis.
Contact / Webpage
Area(s) of Interest
Physical (Ph)
Analytical (An)
Biological (Bi)
Chemical Physics (CP)
Inorganic (In)
Material (Ma)
Selected Publications
2H Nuclear Magnetic Resonance Spectroscopy Supports Larger Amplitude Fast Motion and Interference with Lipid Chain Ordering for Membrane that Contains β Sheet Human Immunodeficiency Virus gp41 Fusion Peptide or Helical Hairpin Influenza Virus Hemagglutinin Fusion Peptide at Fusogenic pH, U. Ghosh and D. P. Weliky, Biochim. Biophys. Acta 2020, 1862, 183404.
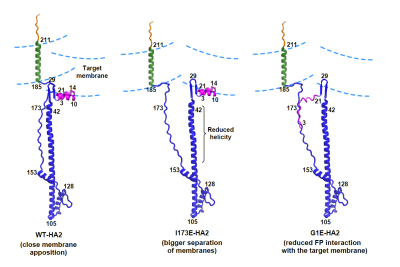
Hydrogen-Deuterium Exchange Supports Independent Membrane-Interfacial Fusion Peptide and Transmembrane Domains in Subunit 2 of Influenza Virus Hemagglutinin Protein, a Structured and Aqueous-Protected Connection between the Fusion Peptide and Soluble Ectodomain, and the Importance of Membrane Apposition by the Trimer-of-Hairpins Structure, A. Ranaweera, P. U. Ratnayake, E. A. P. Ekanayaka, R. Declercq, and D. P. Weliky, Biochemistry 2019, 58, 2432-2466.
The Stabilities of the Soluble Ectodomain and Fusion Peptide Hairpins of the Influenza Virus Hemagglutinin Subunit II Protein Are Positively Correlated with Membrane Fusion, A. Ranaweera, P. U. Ratnayake, and D. P. Weliky, Biochemistry 2018, 57, 5480-5493.
Efficient Fusion at Neutral pH by Human Immunodeficiency Virus gp41 Trimers Containing the Fusion Peptide and Transmembrane Domains, S. Liang, P. U. Ratnayake, C. Keinath, L. Jia, R. Wolfe, A. Ranaweera, and D. P. Weliky, Biochemistry 2018, 57, 1219-1235.
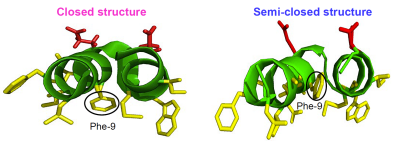
Closed and Semiclosed Interhelical Structures in Membrane vs Closed and Open Structures in Detergent for the Influenza Virus Hemagglutinin Fusion Peptide and Correlation of Hydrophobic Surface Area with Fusion Catalysis, U. Ghosh, L. Xie, L. Jia, S. Liang, and D. P. Weliky, J. Am. Chem. Soc. 2015, 137, 7548-7551.
REDOR Solid-State NMR as a Probe of the Membrane Locations of Membrane- Associated Peptides and Proteins, L. Jia, S. Liang, K. Sackett, L. Xie, U. Ghosh, and D. P. Weliky, J. Mag. Res. 2015, 253, 154-165.
CV
B.A., 1985, Swarthmore College
Ph.D., 1995, Univ. of Chicago
Postdoctoral Fellow, 1995-97, National Institutes of Health.
Awards
| Year | Award | Organization |
|---|---|---|
| 1998 | New Faculty Award | Camille and Henry Dreyfus Foundation |
| 1996 | NIH Fellows Award for Research Excellence | National Institutes of Health |
| 1995 - 1997 | Postdoctoral Research Fellow | National Institutes of Health |
| 1995 | Ph.D. | The University of Chicago |
| 1992 | Research Fellow | AT&T Bell Laboratories |
| 1991 - 1995 | AT&T Ph.D. Scholar | AT&T |
| 1988 - 1991 | NSF Predoctoral Fellow | National Science Foundation |
| 1987 - 1991 | McCormick Fellow | |
| 1985 | Member | Sigma Xi Honor Society (Swarthmore College) |
| 1985 | Phi Beta Kappa | Phi Beta Kappa (Swarthmore College) |
| 1985 | Bachelor of Arts with High Honors in Chemistry and Physics | Swarthmore College |
| 1981 - 1985 | National Merit Scholar | |
| 1981 - 1982 | N. Harvey Collisson Scholar | Swarthmore College |