Timothy H. Warren

Research
Synthetic Inorganic Chemistry for Catalysis and Biology
(Research Description PDF)
We develop environmentally friendly new methods for organic synthesis through ubiquitous C-H bonds, explore the interconversion of nitrogen and ammonia as carbon-free fuels, and decode ways that biology communicates using nitric oxide as a molecular messenger. Our laboratory studies how chemical reactions work that are catalyzed by metal ions such as nickel, copper, and zinc to better enable new insights into the development of useful catalysts for synthesis and energy applications as well as to lay the groundwork for therapeutic interventions connected with nitric oxide misregulation.
Our group works in three areas joined by synthetic inorganic chemistry to understand the structure and reactivity of key intermediates that control C-H functionalization, ammonia/nitrogen interconversion, and bioinorganic nitric oxide processing. We employ a diverse range of synthesis and characterization techniques to reveal insights into chemical structure and reactivity. Beyond more traditional inorganic and organic synthesis techniques, students regularly perform X-ray crystallography, EPR spectroscopy, low temperature UV-vis spectroscopy, and DFT calculations. We also collaborate extensively with scientists from other institutions to study new molecules we make by resonance Raman spectroscopy as well as X-ray absorption and emission spectroscopy.
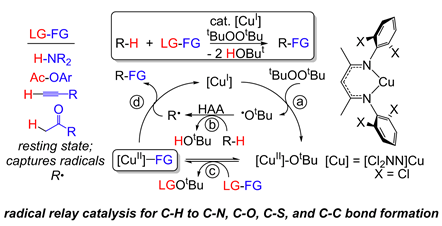
C-H Functionalization
Through mechanistic understanding of reactive copper intermediates, we develop new reactions to directly convert sp3 C-H to C-C, C-N, C-O, and C-S bonds. These methods offer unique synthetic opportunities for the late stage functionalization of organic molecules.
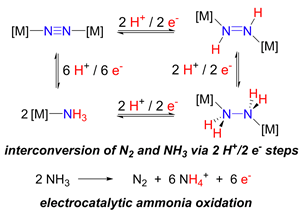
Ammonia – A Carbon-Free Fuel
Ammonia is a carbon-free fuel produced on a scale of over 150 M tons / year. We examine the fundamental chemistry of N-H and N-N bond cleavage and formation to enable the development molecular electrocatalysts based on iron and copper to cleanly generate energy from NH3 and ultimately, to sustainably produce NH3 from N2.
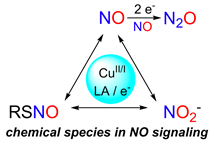
Modeling NO Signaling Chemistry
Nitric oxide (NO) is a powerful signaling molecule with far reaching effects. Inspired by Cu and Zn environments in biology, we employ synthetic models that outline pathways to interconvert molecules in NO signaling such as nitrate, nitrite, and S-nitrosothiols (RSNO).
Contact / Webpage
Area(s) of Interest
Inorganic (In)
Biological (Bi)
Organic (Or)
Organometallic (Om)
Selected Publications
A Three Coordinate Copper(II) Alkynyl Complex in C-C Bond Formation: The Sesquicentennial of the Glaser Coupling, Bakhoda, A.; Okoromoba, O.; Greene, C.; Borougeni, M. R.; Bertke, J. A.; Warren, T.H.* J. Am. Chem. Soc. 2020, 142, 18483-18490.
Lewis Acid Coordination Redirects S-Nitrosothiol Signaling Output, Hosseininasab, V.; McQuilken, A. C.; Bakhoda, A.; Bertke, J. A.; Timerghazin, Q. K.; Warren, T. H. Angew. Chem. Int. Ed. 2020, 59, 10854-10858. Noted as a Very Important Paper
Tris(pyrazolyl)borate Copper Hydroxide Complexes Featuring Tunable Intramolecular H-bonding, Gardner, E. J.; Cobb, C. R.; Bertke, J. A.; Warren, T. H.* Inorg. Chem. 2019, 58, 11248-11255.
Copper-Catalyzed C(sp3)-H Amidation: Sterically Driven Primary and Secondary C-H Site-Selectivity, Bakhoda, A.; Jiang, Q.; Bertke, J. A.; Cundari, T. R.*; Warren, T. H.* Angew. Chem. Int. Ed. 2019, 58, 3421-3425.
Nitrosyl Linkage Isomers: NO Coupling to N2O at a Mononuclear Site, Kundu, S.; Phu, P. N.; Ghosh, P.; Kozimor, S. A.; Bertke, J. A.; Stieber, S. C. E.*; Warren, T. H.* J. Am. Chem. Soc. 2019, 141, 1415-1419.
Copper(II) Activation of Nitrite: Nitrosation of Nucleophiles and Generation of NO by Thiols, Kundu, S.*; Kim, W. Y.; Bertke, J. A.; Warren, T. H.* J. Am. Chem. Soc. 2017, 139, 1045-1048. Highlighted as a JACS Spotlight: Nitric Oxide on a New Route
Copper Catalyzed sp3 C-H Etherification with Acyl Protected Phenols, Salvador, T. K.; Arnett, C. H.; Kundu, S.; Sapiezynski, N. G.; Bertke, J. A.; Boroujeni, M. R.; Warren, T. H.* J. Am. Chem. Soc., 2016, 138, 16580-16583.
A Motif for Reversible Nitric Oxide Interactions in Metalloenzymes, Zhang, S.; Melzer M. M.; Nermin Sen, S.; Çelebi-Ölçüm, N.*; Warren, T. H.* Nature Chem. 2016, 8, 663-669.
CV
B.S., 1992, University of Illinois at Urbana-Champaign
Ph.D., 1997, Massachusetts Institute of Technology
Postdoctoral Fellow, 1997-1999, University of Münster
1999 – 2021 Professor, Georgetown University