Milton R. (Mitch) Smith

Research
Inorganic and Organic Chemistry for Sustainability in the 21st Century
(Research Description PDF)
Our research ranges from developing metal-catalyzed reactions that act on organic and inorganic substrates, to designing polymers that interact in interesting ways with enzymes and drugs. The connecting theme is developing new chemistry and mentoring the next generation of scientists to tackle the challenge of sustainably meeting the needs of our species, which is predicted to number 9.5-13 billion people by the year 2100. We have several ongoing projects and frequently collaborate with scientists in academia and industry.
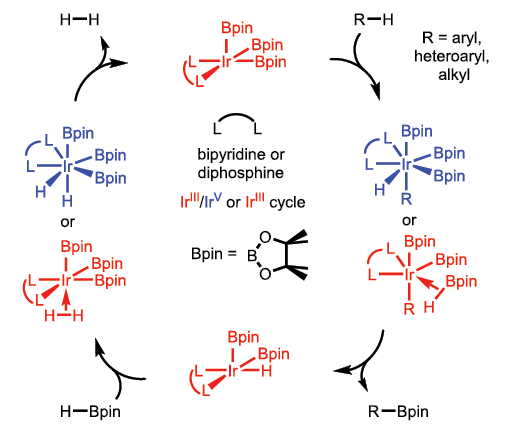
We have had long-standing interest in reactions of metal boryl complexes (M–BZ2, where Z is an anionic substituent such as alkoxide) with organic molecules. Organoboron compounds are used extensively in pharmaceutical, agrochemical, and advanced materials industries. Consequently, new chemistry within this molecular class can eliminate steps and reduce waste streams associated with synthesis of these valuable intermediates. In this regard, we have developed new catalytic reactions, such as additions of B–B bonds to olefins and synthesis of B–C bonds from hydrocarbons and boranes, etc. The first thermal example of the latter catalytic reaction, now commonly referred to as C–H borylation, was discovered in our group. More recently, mechanistic studies integrating experimental and computational approaches have given us a deeper understanding of C–H borylation. The computational work is inspiring the design of new catalysts and boron reagents that are tailored to specific substrate classes.
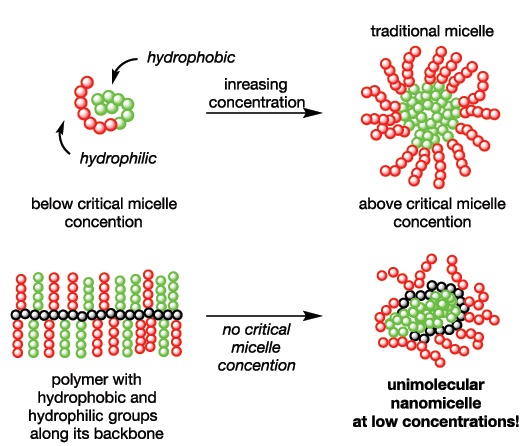
Our early polymer work focused on synthesis of biodegradable polymers with controllable properties. This evolved into the design of monomers with functional groups, which gave polymers that could readily modified through chemical reactions. Recently, we have found that by incorporating combinations of hydrophobic and hydrophilic groups along the backbone, polymers that behave as nanomicelles can be prepared. Since the length of the initial polymer, and the sizes of hydrophilic and hydrophobic groups that are subsequently introduced, determine the sizes of the nanomicelles, we are exploring host-guest chemistry of these materials. For example, we have found that the nanomicelles can interact with enzymes to make them soluble in organic solvents with retention of enzymatic activity.
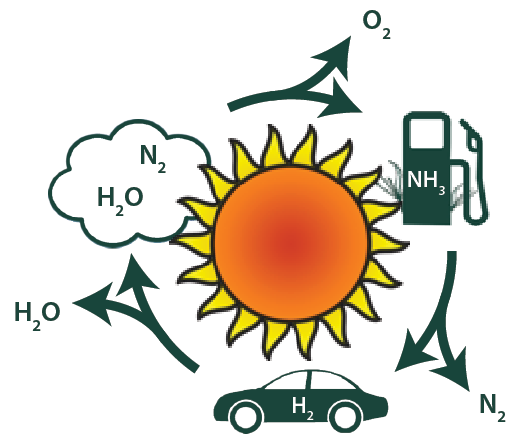
The newest project in our group explores the synthesis of ammonia and related nitrogen compounds using renewable energy. Ammonia synthesis via the Haber-Bosch process currently consumes approximately 2% of the energy we produce. The H2 that is required in the Haber-Bosch synthesis is produced by reacting methane or coal with water, which also generates large quantities of CO2. Our goal is to develop new approaches for NH3 synthesis that can be coupled effectively to renewable energy sources like solar and wind, whose availability is intermittent.
The flip side of this project is that ammonia and other nitrogen compounds like hydrazine have been used as fuels. In fact, the X-15 aircraft that still holds speed and altitude records was fueled by NH3. An example of a renewable nitrogen-based fuel cycle is shown below.
Webpage
Area(s) of Interest
Inorganic (In)
Biological (Bi)
Material (Ma)
Organometallic (Om)
Selected Publications
Catalytic borylation of methane, Smith, K. T.; Berritt, S.; González-Moreiras, M.; Ahn, S.; Smith, M. R., III; Baik, M.-H.; Mindiola, D. J., Science 2016, 351, 1424-1427.
Dextran functionalization enhances nanoparticle-mediated siRNA delivery and silencing, Vocelle, D.; Chesniak, O. M.; Malefyt, A. P.; Comiskey, G.; Adu-Berchie, K.; Smith, M. R., III; Chan, C.; Walton, S. P., Technology 2016, 04, 42-54.
Electrolysis of liquid ammonia for hydrogen generation, Little, D. J.; Smith, M. R., III; Hamann, T. W., Energy Environ. Sci. 2015, 8, 2775-2781.
Reversible Borylene Formation from Ring-Opening of Pinacolborane and Other Intermediates Generated From 5-Coordinate Trisboryl Complexes: Implications for Catalytic C–H Borylation, Ghaffari, B.; Vanchura, B. A., II; Chotana, G. A.; Staples, R. J.; Holmes, D.; Maleczka, R. E., Jr.; Smith, M. R., III, Organometallics 2015, 34, 4732-4740.
Harnessing C–H Borylation/Deborylation for Selective Deuteration, Synthesis of Boronate Esters, and Late Stage Functionalization, Kallepalli, V. A.; Gore, K. A.; Shi, F.; Sanchez, L.; Chotana, G. A.; Miller, S. L.; Maleczka, R. E., Jr.; Smith, M. R., III, J. Org. Chem. 2015, 80, 8341-8353.
Silyl Phosphorus and Nitrogen Donor Chelates for Homogeneous Ortho Borylation Catalysis, Ghaffari, B.; Preshlock, S. M.; Plattner, D. L.; Staples, R. J.; Maligres, P. E.; Krska, S. W.; Maleczka, R. E., Jr.; Smith, M. R., III, J. Am. Chem. Soc. 2014, 136, 14345-14348.
A Traceless Directing Group for C–H Borylation, Preshlock, S. M.; Plattner, D. L.; Maligres, P. E.; Krska, S. W.; Maleczka, R. E., Jr.; Smith, M.R., III, Angew. Chem., Int. Ed. 2013, 52, 12,915-12,919.
"Clickable" polyglycolides: Tunable synthons for thermoresponsive, degradable polymers, Jiang, X.; Vogel, E. B.; Smith, M. R., III; Baker, G. L., Macromolecules 2008, 41, 1937-1944.
CV
B.S., 1986, California Institute of Technology
Ph.D., 1990, Univ. of Chicago
Postdoctoral Fellow, 1990-92, Univ. of California, Berkeley.
Awards
| Year | Award | Organization |
|---|---|---|
| 2019 | American Chemical Society (ACS) Award in Organometallic Chemistry | |
| 2016 | Outstanding Faculty Award | Michigan State University (College of Natural Science) |
| 2016 | William J. Beal Outstanding Faculty | Michigan State University |
| 2015 | Outstanding Faculty Award | College of Natural Science |
| 2013 | Merck Technology Symposium's Technology Collaboration Award | |
| 2008 | Distinguished Service in Boron Science Award | |
| 2008 | US EPA’s Presidential Green Chemistry Challenge Award | |
| 1990 | Ph.D. | University of Chicago |
| 1990 - 1992 | Postdoctoral Fellow | University California, Berkeley |
| 1986 | Bachelor of Science | California Institute of Technology |