Thomas O'Halloran

Research
Chemistry
(Research Description PDF)
The O’Halloran laboratory focuses on de-veloping a quantitative understanding of the regulatory roles of transition metals in controlling cellular physiology. Each cell must acquire millions (bacteria) to billions (eukaryotes) of metal ions, monitor, and balance the levels of these ions to prevent excessive accumulation while simultaneously keeping metals flowing into essential catalytic and signaling processes. My highest priority is training the next generation of scientists to become rigorous and creative experts who can guide discovery at the interface of the physical and biological sciences. The trans-disciplinary training in my lab both prepares and enables students to solve some of the most challenging and pressing problems in chemical, biological, and biomedical research.
Quantitative Inorganic Physiology. Imagine being able to count the atoms of the essential elements from the single-cell level, in subcellular compartments, and at the tissue-level. Then imagine measuring the changes in the distribution and activity of metal ions in response to environmental and developmental signals as cellular cues for fundamental processes. This nascent field of inorganic physiology intersects the traditional disciplines of chemistry and biology and harmonizes with cutting-edge elemental mapping technologies developed with physicists and analytical chemists at the helm. We are developing quantitative analytical techniques along several thrusts, including single-cell mass spectrometry, X-ray fluorescence microscopy, and laser ablation time-of-flight mass spectrometry.
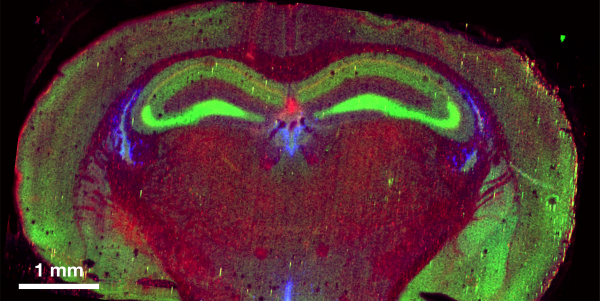
Regulatory Metal Fluxes Controlling Cell Cycle Progression. Using our new zinc-specific probes and synchrotron X-ray fluorescence microscopy, we developed novel imaging methods and discovered that zinc fluxes in the mammalian egg regulate both the exit from the meiotic cell cycle and the resumption of mitosis upon fertilization. Most strikingly, fertilization of a mammalian egg initiates a series of ‘zinc sparks’, rapid exocytosis of over 10 billion zinc ions, that are necessary to induce the egg-to-embryo transition. Very recently, we have discovered that zinc sparks are not only conserved in the amphibian Xenopus laevis, but fertilization also triggers a loss of intracellular manganese to prevent polyspermy.
Biological Inorganic Chemistry of Cancer. Insights into the inorganic chemistry of the cell have led to the development of new therapeutic agents. Cisplatin and arsenic trioxide are anti-cancer drugs that both induce apoptotic cell death, but through different biochemical pathways. To improve the efficacies of these two drugs, the O’Halloran group utilized a nanomaterials approach to co-encapsulate high levels of arsenous acids and aqua-cisplatin into 100 nm liposomes that are stable in serum but release their drug in the low-pH endosome of tumor cells. In a separate coordination chemistry approach, we developed arsenoplatin, an arsenous acid-platinum complex that shows significant biological activity in several cancer cell lines and with activity distinct from cisplatin and arsenic trioxide individually.
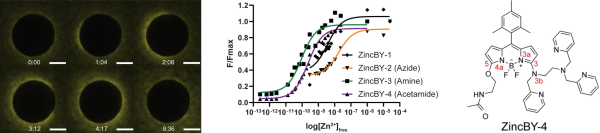
Metal fluxes controlling fertilization. Left: live-cell fluorescence zinc imaging demonstrates that fertilization of X. laevis oocytes induces a zinc spark, which was first observed in mammalian models (Seeler et al., Nat. Chem. 2021). Middle and Right: to quantify and map labile pools of metals, our lab develops metal-responsive probes, such as the ZincBY family of zinc-responsive fluorescent probes (Garwin et al. J. Am. Chem. Soc. 2019).
Contact / Webpage
Area(s) of Interest
Inorganic (In)
Analytical (An)
Biological (Bi)
Selected Publications
Metal Ion Fluxes Controlling Amphibian Fertilization, Seeler, J.F.; Sharma, A.; Zaluzec, N.J.; Bleher, R.; Lai, B.; Schultz, E. G.; Hoffman, B.M.; LaBonne, C.; Woodruff, T. K.; O’Halloran, T. V., Nat. Chem. 2021, 13, 683–691.
CueR Activates Transcription through a DNA Distortion Mechanism, Fang, C.; Philips, S.J.; Wu, X.; Chen, K.; Shi, J.; Shen, L.; Xu, J.; Feng, Y.; O’Halloran, T.V.; Zhang, Y. , Nat. Chem. Biol. 2021, 17, 57–64.
Interrogating Intracellular Zinc Chemistry with a Long Stokes Shift Zinc Probe ZincBY-4, Garwin, S.A.; Kelley, M.S. J.; Sue, A.C.; Que, E. L.; Schatz, G.C.; Woodruff, T.K.; O’Halloran, T.V., J. Am. Chem. Soc. 2019, 141, 16696–16705.
Arsenoplatin-1 Is a Dual Pharmacophore Anticancer Agent, Miodragović, Đ.; Merlino, A.; Swindell, E.P.; Bogachkov, A.; Ahn, R. W.; Abuhadba, S.; Ferraro, G.; Marzo, T.; Mazar, A.P.; Messori, L.; O’Halloran, T.V., J. Am. Chem. Soc. 2019, 141, 6453–6457.
Allosteric Transcriptional Regulation via Changes in the Overall Topology of the Core Promoter, Philips, S.J.; Canalizo-Hernandez, M.; Yildirim, I.; Schatz, G.C.; Mondragon, A.; O’Halloran, T.V., Science 2015, 349, 877–881.
Quantitative Mapping of Zinc Fluxes in the Mammalian Egg Reveals the Origin of Fertilization-Induced Zinc Sparks, Que, E. L.; Bleher, R.; Duncan, F.E.; Kong, B.Y.; Gleber, S.C.; Vogt, S.; Chen, S.; Garwin, S. A.; Bayer, A.R.; Dravid, V.P.; Woodruff, T.K.; O’Halloran, T.V., Nat. Chem. 2015, 7, 130–139
CV
B.S., M.S., 1980, Univ. of Missouri
Ph.D., 1985,Columbia Univ.
Postdoctoral Fellow, 1986, Massachusetts Inst. of Technology
Awards
| Year | Award | Organization |
|---|---|---|
| 2020 | Elected Fellow | National Academy of Inventors |
| 2016 | The Annual Dewitt Stetten Jr. Lecture | NIH National Institute of General Medical Science |
| 2013 | Royal Society of Chemistry Dalton Award for Bioinorganic Chemistry | Royal Society of Chemistry Dalton Section |
| 2013 | Fellow, Royal Society of Chemistry | Royal Society of Chemistry |
| 2008 | David Denks Award for Research on Copper Homeostasis & its Disorders | 6th International Copper Meeting |
| 2007 | Elected Fellow | Japanese Society for Promotion of Science |
| 2001 - 2010 | MERIT Award | NIH |
| 1999 | Elected Fellow | American Association for the Advancement of Science |
| 1998 - 1999 | Fellow | John Simon Guggenheim Memorial Foundation |
| 1996 | Faculty Honor Roll | Faculty Honor Roll (Associated Student Government, Northwestern University) |
| 1996 | Scientific Achievement Award | American Society for Biochemistry and Molecular Biology; Sponsored by Schering-Plough |
| 1993 - 1995 | Camille and Henry Dreyfus Teacher-Scholar Award | The Camille and Henry Dreyfus Foundation |
| 1991 - 1993 | Fellow | Alfred P. Sloan Research |
| 1989 - 1991 | Biochemistry Award | Eli Lilly |
| 1987 - 1992 | Presidential Young Investigator Award | NSF |
| 1987 - 1990 | National Searle Scholars Award | The Chicago Community Trust |
| 1986 | Distinguished New Faculty Award | The Camille and Henry Dreyfus Foundation |
| 1986 - 1987 | Du Pont Young Faculty Award | Northwestern University |
| 1985 - 1986 | National Research Service Award Postdoctoral Fellowship | NIH |
| 1984 | Pegram Award | Columbia University, Ph.D. program |
| 1980 | Full Member | Society of the Sigma Xi |