Kenneth Merz

Research
Computational Approaches to Biomolecular Systems
(Research Description PDF)
Research at the interface between the computational sciences and biology is our group focus. We work on a number of problems and collaborate with experimentalists at every opportunity. Research areas of most interest include computer-aided drug design (CADD), the role potential function error plays in drug design and protein folding, metalloenzymes and metal ion homeostasis, development and application of linear-scaling quantum mechanical methods to biological problems and NMR and X-ray structure refi ment using quantum mechanical methods. For further details go to the group web page at http://merzgroup.org.
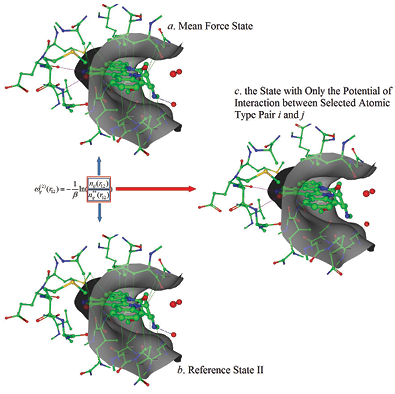
In the structure-based drug design area we are interested in developing novel tools to predict the binding affi y of ligands for a given receptor. Along these lines we developed a novel knowledge-based protein-ligand scoring function that employs a new defi ition for the reference state (see the Figure), allowing us to relate a statistical potential to a LennardJones (LJ) potential. In this way, the LJ potential parameters were generated from protein-ligand complex structural data contained in the PDB. Forty-nine types of atomic pairwise interactions were derived using this method, which we call the knowledge-based and empirical combined scoring algorithm (KECSA). Validation results illustrate that KECSA shows improved performance in all test sets when compared with other scoring methods especially in its ability to minimize the RMSE.
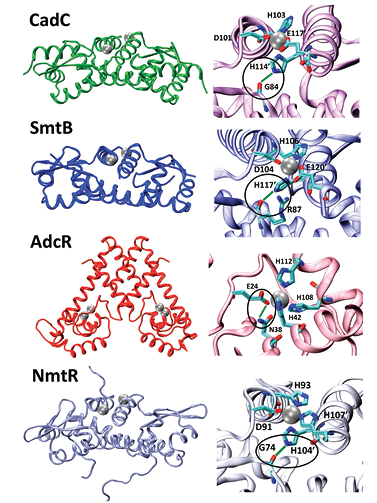
Metalloenzymes carry out a myriad of biological functions and we have a long-term interest in modeling the structure and function of proteins involving metal ion catalysis or homeostasis. A recent publication illustrates an example of the study of transition metal homeostasis. A metal-mediated interprotomer hydrogen bond has been implicated in the allosteric mechanism of DNA operator binding in several metal-sensing proteins. Using computational methods, we investigated the energetics of such zinc-mediated interactions in members of the ArsR/SmtB family of proteins (CzrA, SmtB, CadC and NmtR) and the MarR family zinc-uptake repressor AdcR, each of which feature similar interactions, but in sites that differ widely in their allosteric responsiveness. We provided novel structural insight into previously uncharacterized allosteric forms of these proteins using computational methodologies. We find this metal-mediated interaction to be significantly stronger (~8 kcal/mol) at functional allosteric metal binding sites compared to a non-responsive site (CadC) and the apo-proteins. Simulations of the apo-proteins further revealed that the high interaction energy works to overcome the considerable disorder at these hydrogen-bonding sites and functions as a “switch” to lock in a weak DNA-binding conformation once metal is bound. These fi suggested a globally conserved functional role of metal-mediated second-coordination shell hydrogen bonds at allosterically responsive sites in zinc-sensing transcription regulators.
Contact / Webpage
Area(s) of Interest
Physical (Ph)
Biological (Bi)
Theoretical and Computational (Th)
Selected Publications
Thermodynamics of Transition Metal Ion Binding to Proteins, L. Song; A. Sengupta; K. M Merz Jr., J. Am. Chem. Soc. 2020, 142, 6365-6374.
Random Forest Refinement of Pairwise Potentials for Protein-ligand Decoy Detection, J. Pei; Z. Zheng; H. Kim; L. Song; S. Walworth; M. Merz; K. M. Merz, Jr., J. Chem Inf. Model. 2019, 59, 3305-3315.
Random Forest Refinement of the KECSA2 Knowledge-based Scoring Function for Protein Decoy Detection, J. Pei; Z. Zheng; K. M. Merz, Jr., J. Chem Inf. Model. 2019, 59, 1919-1929.
Using AMBER18 for Relative Free Energy Calculations, L. Song; T.S. Lee; C. Zhu; D.M. York; K. M. Merz, Jr., J. Chem Inf. Model. 2019, 59, 3128-3135.
Trapping intermediates in metal transfer reactions of the CusCBAF export pump of Escherichia coli, K.N. Chacón; J. Perkins; Z. Mathe; K Alwan; E.N. Ho; M.N. Ucisik; K. M. Merz, Jr.; N. J. Blackburn, Communications Biology 2018, 1, 192.
Simulating the Chelate Effect, A. Sengupta; A. Seitz; K. M. Merz, Jr., J. Am. Chem. Soc. 2018, 140, 15166-15169.
CV
B.S., 1981, Washington College
Ph.D., 1985, Univ. of Texas at Austin
Post-Doctoral Fellow, 1986-87, Cornell Univ.
Post-Doctoral Fellow, 1987-89, Univ. of California at San Francisco
Awards
| Year | Award | Organization |
|---|---|---|
| 2021 | University Distinguished Professor |