Robert E. Maleczka

Research
Synthetic Organic Chemistry
(Research Description PDF)
Our group is interested in a) green chemistry, b) the invention of new reactions and strategies in organic synthesis, and c) target synthesis of molecules with interesting properties ranging from biologically important natural products to nanomaterials.
Green Chemistry: Central to our research is the development of efficient and environmentally benign reactions and strategies. The Pharmaceutical Roundtable of the American Chemical Society’s Green Chemistry Institute deemed cross-couplings that avoid haloaromatics as their top aspirational reaction. In collaboration with Professor Mitch Smith, we are inventing such reactions. Specifically, we are using catalytic C–H activation/borylation, often combined with subsequent chemical events, to generate pharmaceutically relevant building blocks for organic synthesis and the late stage functionalization of drugs and drug candidates.
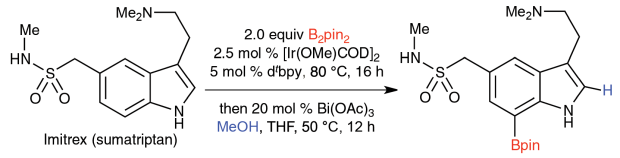
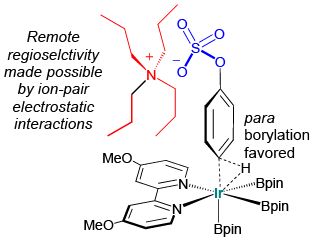
Invention of New Reactions: The principles of green chemistry also motivate us to create new synthetic methods. Within our catalytic borylation program, an example is our recently disclosed use of ion-pairing, where the alkyl groups of the cation create a steric shield that facilitates previously unheard of levels of para selectivity.
We are also focusing on the employment of organosilanes as both reagents and substrates in chemical transformations ranging from Wittig rearrangements to new approaches to double-decker silsesquioxanes (DDSQ’s) for materials applications.
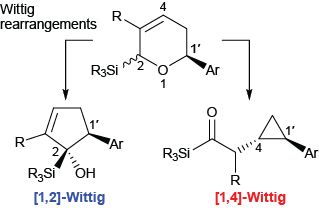
Since their discovery more than 70 years ago, Wittig rearrangements have evolved into powerful tools for the isomerization of a-metalated ethers into alkoxides. Relative to the [2,3]- and [1,2]-shifts, [1,4]-Wittig rearrangements are unique in their ability to generate stereodefined enolates. In addition, [1,4]-Wittig rearrangements have the potential to transfer chirality and stereoselectively form adjacent chiral centers. As such, we continue to study these mechanistically fascinating and synthetically intriguing rearrangements.
We have also teamed up with Chemical Engineering and Materials Science Professor Andre Lee, to apply our synthetic expertise to another remarkable class of compounds, namely silsesquioxanes. These caged structures have garnered significant attention over their ability to meet the demands of medical, aerospace and materials industries. This is due to the well-defined spatial dimensions, the presence of seven inert peripheral organic moieties to accommodate solubility and processability, and one polymerizable reactive organic group.
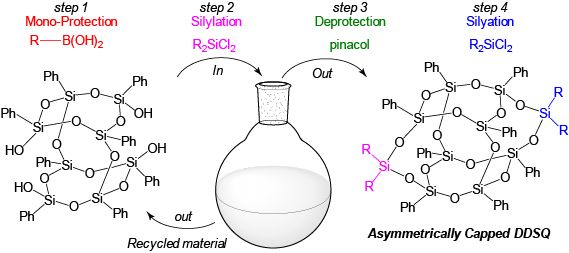
Like our other projects, we approach the synthesis of silsesquioxane through the lens of green chemistry. An example of this can be seen in our development of the first direct synthetic route leading to asymmetrically functionalized DDSQ compounds. By way of our route over 50% of the starting DDSQ tetraol that could have otherwise contributed toward the synthesis of unwanted side products is recovered with a high purity and can be used in another cycle of synthesis. Efforts to use these compounds as nano-linkers to two different block copolymers are underway in our lab.
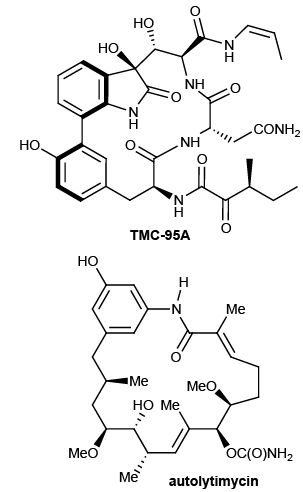
Target Synthesis: A unifying thesis behind all of our methodological and mechanistic studies is that the chemistry to emerge from such studies should be applicable to real synthetic problems. We view target synthesis as the best proof of this concept. For example, as part of our green chemistry program, we look to make TMC-95A and autolytimycin by the strategic application of our own synthetic methods.
Contact / Webpage
Area(s) of Interest
Organic (Or)
Organometallic (Om)
Selected Publications
“Steric shielding effects induced by intramolecular C–H•••O hydrogen bonding: Remote borylation directed by Bpin groups” Montero Bastidas, J. R.; Chhabra, A.; Feng, Y.; Oleskey, T. J.; Smith, M. R., III; Maleczka, R. E.; Jr. ACS Catal. 2022, 12, 2694–2705.
“Merging Iridium-Catalyzed C–H Borylations with Palladium-Catalyzed Cross-Couplings using Triorganoindium Reagents” Jayasundara, C. R. K.; Gil-Negrete, J.-M.; Montero Bastidas, J. R.; Chhabra, A.; Martínez, M. M.; Sestelo, J. P.; Smith, M. R., III; Maleczka, R. E.; Jr. J. Org. Chem. 2022, 87, 751–759.
“Amide Directed Iridium C(sp3)–H Borylation Catalysis with High N-Methyl Selectivity” Dannatt, J. E.; Yadav, A.; Smith, M. R., III; Maleczka, R. E.; Jr. Tetrahedron 2022, 109, 132578.
“Silylcyclopropanes by Selective [1,4]-Wittig Rearrangement of 4-Silyl-5,6-Dihydropyrans” Mori-Quiroz, L. M.; Maloba, E. W.; Maleczka, R. E.; Jr. Org. Lett. 2021, 23, 5724–5728.
“Phase behavior of selected condensed double-decker shaped silsesquioxane compounds” Vogelsang, D. F.; Maleczka, R. E., Jr.; Lee, A. Silicon 2021, 13, published on-line 11/4/2021.
One-Pot Iridium Catalyzed C–H Borylation/ Sonogashira Cross-Coupling: Access to Borylated Aryl Alkynes, Chotana, G. A.; Montero Bastida, J. R.; Miller, S. L.; Smith, M. R., III; Maleczka, R. E., Jr., Molecules 2020, 25, 1754–1766.
Para-Selective, Iridium-Catalyzed C–H Borylations of Sulfated Phenols, Benzyl Alcohols, and Anilines Directed by Ion-Pair Electrostatic Interactions, M. Bastida, J. R.; Oleskey, T. J.; Miller, S. L.; Smith, M. R., III; Maleczka, R. E., Jr., J. Am. Chem. Soc. 2019, 141, 15483–15487.
C–H Borylation Catalysts that Distinguish Between Similarly Sized Substituents Like Fluorine and Hydrogen, Miller, S. L.; Chotana, G. A.; Fritz, J. A.; Chattopadhyay, B.; Maleczka, R. E., Jr.; Smith, M. R., III, Org. Lett. 2019, 21, 6388–6392.
A General Diversity Oriented Synthesis of Asymmetric Double-Decker Shaped Silsesquioxanes, Barry, B.-D.; Dannatt, J. E.; King, A. K.; Lee, A.; Maleczka, R. E., Jr., Chem. Commun. 2019, 55, 8623–8626.
Phase Behavior of cis–trans Mixtures of Double-Decker Shaped Silsesquioxanes for Processability Enhancement, Vogelsang, D. F.; Dannatt, J. E.; Shoen, B. W.; Maleczka, R. E., Jr.; Lee, A., ACS Appl. Nano Mater. 2019, 2, 1223–1231.
Predictive Liquid Chromatography Separation for Mixtures of Functionalized Double-decker Shaped Silsesquioxanes Based on HPLC Chromatograms, Vogelsang, D. F.; Maleczka, R. E., Jr.; Lee, A., Ind. Eng. Chem. Res. 2019, 58, 403–410.
HPLC Characterization of cis and trans Mixtures of Double-Decker Shaped Silsesquioxanes, Vogelsang, D. F.; Maleczka, R. E., Jr.; Lee, A., Silicon 2019, 11, 5–13.
CV
B.S., 1984, Univ. of Illinois at Urbana-Champaign
Abbott Labs, 1984-1987
Ph.D., 1992, The Ohio State Univ.
Postdoctoral Fellow, 1992-1995, American Cancer Society Univ. of Pennsylvania
Awards
| Year | Award | Organization |
|---|---|---|
| 2021 | Elected Fellow of the American Association for the Advancement of Science (AAAS) | |
| 2018 | Named Honored Professor of ‘Overseas Expertise Introduction Center for Green Pharmaceuticals Discipline Innovation (111 Center)’ | Zhejiang University of Technology, China |
| 2018 | Hess Lecturer | Illinois Wesleyan University |
| 2014 | Elected Fellow of the American Chemical Society (ACS) | |
| 2013 | Meritorious Faculty Award by the College of Natural Science Alumni Association | |
| 2013 | Merck Technology Collaboration Award | Merck |
| 2008 | US EPA’s Presidential Green Chemistry Challenge Award | |
| 2006 | Astellas USA Foundation Faculty Award | Astellas USA Foundation |
| 2003 - 2004 | Smith First Edition Board of Advisors | McGraw Hill |
| 2001 - 2005 | Yamanouchi USA Foundation Faculty Award | Yamanouchi USA Foundation |
| 2001 | Teacher-Scholar Award | College of Natural Science |
| 2000 | Novartis Lecturer | Yale University |
| 2000 - 2003 | NSF Career Award | National Science Foundation |
| 2000 | Meritorious Faculty Research Award | Michigan State University |
| 2000 | Junior Faculty Award | Sigma Xi Honor Society (Michigan State University) |
| 1998 - 2001 | NIH First Award | National Institutes of Health |
| 1998 | MSU Chapter Mentor of the Year | National Organization for the Professional Advancement of Black Chemists and Chemical Engineers |
| 1998 | MSU Senior Class Council Outstanding Faculty Award Nominee | Michigan State University (Senior Class Council) |
| 1993 - 1995 | Postdoctoral Fellow | American Cancer Society |
| 1993 | American Cancer Society Post-Doctoral Fellow | University of Pennsylvania |
| 1987 - 1992 | Graduate Research Associate | The Ohio State University |
| 1984 - 1987 | Research Assistantship | Abbott Laboratories |
| 1984 | Bachelor of Science | University of Illinois, Urbana-Champaign |
| 1983 - 1984 | Undergraduate Research | University of Illinois, Urbana-Champaign |
| 1980 - 1982 | Air Force ROTC Scholarship | Air Force ROTC |
| Dean's List | University of Illinois, Urbana-Champaign |