James E. (Ned) Jackson

Research
Mechanism and Design in Green and Organic Materials Chemistry
(Research Description PDF)
Probing mechanisms and theory, from molecular interactions to process design, Jackson group efforts range from fundamental... :
- Hydrogen bond insights, including hydridic-to-protonic and aromaticitymodulated systems1-3
- Computational modeling to design and interpret reaction mechanisms and structures4-6
...to eminently practical chemistry:
- “Green” catalysts and pathways from renewables to useful “petro-” chemicals7-10
- Studies of bio-relevant solvents to connect molecular interactions to engineering properties.11
More information can be found at www2.chemistry.msu.edu/faculty/jackson/; two active areas are outlined below, where the common thread is mechanistic. By understanding molecular interactions and reactions we seek rules to design materials and processes with targeted characteristics. From the post-doc to the high-school level, scientists trained in the group have gone on to excellent positions in academics, industry, or governmental research.
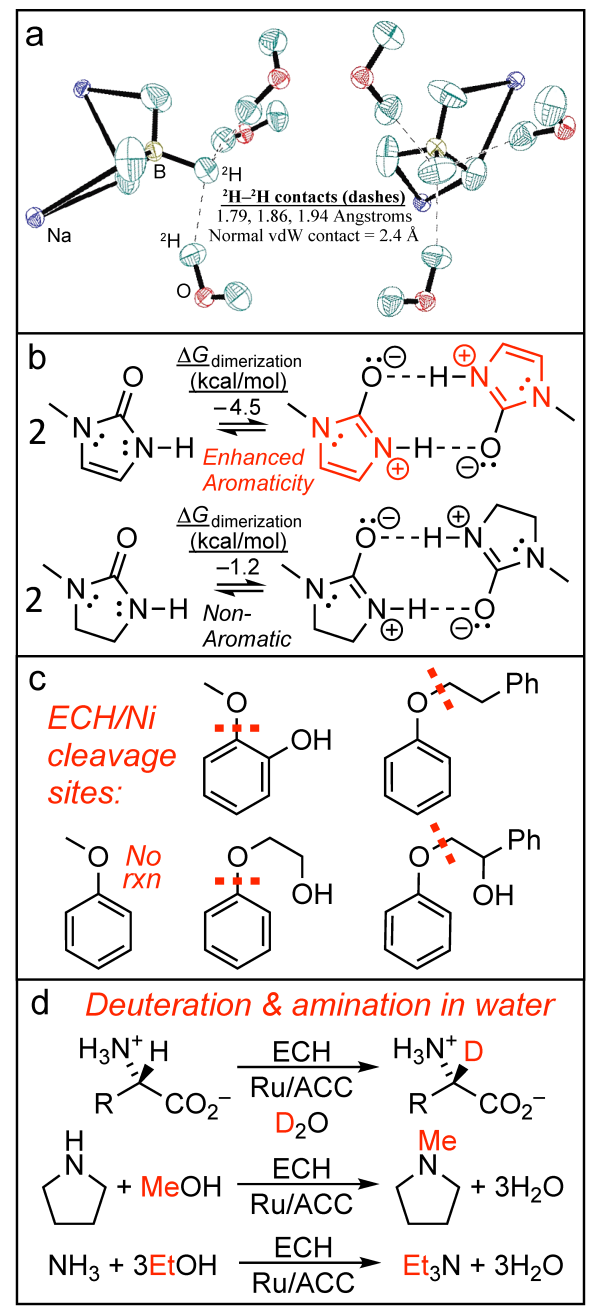
Novel Hydrogen Bonds: Our discovery and studies of hydridic-to-protonic H-bonding, AKA dihydrogen bonding, began with a HS student studying NaBH4•2H2O (Fig. 1a) and includes crystal engineering, searches for bio- and synthesis relevance, and infraredactivated bond-selective reactions.1 We have also uncovered aromaticity/antiaromaticitymodulation of common H-bond strengths (AMHB, Fig. 1b) and have begun collaborative studies with chemical engineers to use our molecular-level understanding of H-bonding to develop practical models for properties and separations of alcohols and other bio-relevant liquids.2-3
Green Chemistry: We seek to replace fossil petroleum with renewables to make chemicals and fuels via catalytic paths starting from biomass feedstocks.7-9 Finite renewable carbon supplies call for C-retentive upgrading (i.e. reduction) of low-value lignin with energy from non-fossil sources. All these (wind, solar, hydro, nuclear) make electric power, so upgrading via electrocatalytic hydrogenation (ECH) is a focus, and has also turned up some novel ether cleavage and C-H activations (Fig. 1c). Having also uncovered very mild electrocatalytic C-H activation chemistry at HOC-H and R2NC-H sites in various molecules, we have now demonstrated that this activation process can be harnessed to use alcohols to alkylate amines, forming water as byproduct (Fig. 1d). Meanwhile, mechanistic studies aid in design/optimization of catalysts and conditions.7-10
Synergy: Our catalytic and electrocatalytic reductions of bio-derived feedstocks in water (practical) and our C-H activation/amination chemistry now intersect with the dihydrogen bond (fundamental) work; interfacial dihydrogen bonding of metal-bound hydride sites under water may tune their reactivity. In turn, our AMHB studies (fundamental) are helping to inform the engineering solvent work (practical).11 Such synergies between practical and fundamental; synthesis, structure and mechanism; and experiment and theory pull us back to the lab each day.
Contact / Webpage
Area(s) of Interest
Organic (Or)
Inorganic (In)
Material (Ma)
Theoretical and Computational (Th)
Selected Publications
- Dihydrogen Bonding: Structures, Energetics, and Dynamics, Custelcean, R.; Jackson, J. E., Chem. Rev. 2001, 101, 1963-1980.
- (Anti)aromaticity-Modulated Hydrogen Bonding, Kakeshpour, T.; Wu, J. I.; Jackson, J. E., J. Am. Chem. Soc. 2016, 138, 3427−3432.
- High-Field NMR Spectroscopy Reveals Aromaticity-Modulated Hydrogen Bonding (AMHB) in Heterocycles, Kakeshpour, T.; Bailey, J. P.; Jenner, M. R.; Howell, D. E.; Staples, R. J.; Holmes, D.; Wu, J. I.; Jackson, J. E., Angew. Chem. Int. Ed. 2017, 56, 9843-9846.
- A New Tool To Guide Halofunctionalization Reactions: The Halenium Affinity (HalA) Scale, Ashtekar, K. D.; Marzijarani, N. S.; Jaganathan A.; Holmes, D.; Jackson, J. E.; Borhan, B., J. Am. Chem. Soc. 2014, 136, 13355-13362.
- Mechanisms and time-resolved dynamics for trihydrogen cation (H ) formation from organic molecules in strong laser fields, Ekanayake, N.; Nairat, M.; Kaderiya, B.; Feizollah, P.; Jochim, B.; Severt, T.; Berry, B.; Kanaka, R. P.; Carnes, K. D.; Pathak, S.; Rolles, D.; Rudenko, A.; Ben- Itzhak, I.; Mancuso, C. A.; Fales, B. S.; Jackson, J. E.; Levine, B. G.; Dantus, M., Sci. Rep. 2017, 7, Art. 4703.
- Absolute and relative facial selectivities in organocatalytic asymmetric chlorocyclization reactions, Salehi Marzijarani, N; Yousefi, R.; Jaganathan, A.; Ashtekar, K.; Jackson, J. E.; Borhan, B., Chem. Sci. 2018, 9, 2898-2908.
- Electrocatalytic Upgrading of Model Lignin Monomers with Earth Abundant Metal Electrodes, Lam, C.H.; Lowe, C.B.; Li, Z.; Longe, K.N.; Rayburn, J.T.; Caldwell, M.A.; Houdek, C.E.; Maguire, J.B.; Saffron, C.M.; Miller, D.J.; Jackson, J.E., Green Chem. 2015, 17, 601-609.
- Towards sustainable hydrocarbon fuels with biomass fast pyrolysis oil and electrocatalytic upgrading, Lam, C. H.; Das, S.; Erickson, N. C.; Hyzer, C. D.; Garedew, M.; Anderson, J. E.; Wallington, T. J.; Tamor, M. A.; Jackson, J. E.; Saffron, C. M., Sustainable Energy Fuels 2017, 1, 258-266 (cover article).
- Stereoretentive H/D exchange via an electroactivated heterogeneous catalyst at sp3 C-H sites bearing amines or alcohol, Bhatia, S.; Spahlinger, G.; Bukhumseen, N. Boll, Q.; Li, Z.; Jackson, J. E., Eur. J. Org. Chem. 2016, 4230-4235.
- Structural and morphological evaluation of Ru-Pd bimetallic nanocrystals, Ma, X.; Lin, R.; Ofoli, R. Y. Mei, Z.; Jackson, J. E., Mater. Chem. Phys. 2016, 173, 1-6.
- https://icer.msu.edu/about/announcements/ icer-student-highlights-aseel-bala
https://icer.msu.edu/about/announcements/ icer-student-highlights-Tayeb-Kakeshpour
CV
A.B., 1977, Harvard Univ.
Ph.D., 1987, Princeton Univ.
Postdoctoral Fellow, 1986-1988, Ohio State Univ.
Awards
| Year | Award | Organization |
|---|---|---|
| 2021 | Undergraduate Teaching Award, College of Natural Science | Michigan State University |
| 2001 | Outstanding Faculty Award Nominee | Michigan State University (ASMSU) |
| 1994 - 1995 | Teacher-Scholar Award | Michigan State University (College of Natural Science) |
| 1993 | Nominated for Teacher-Scholar Award | Michigan State University |
| 1988 | New Faculty Award | Camille and Henry Dreyfus Foundation |
| 1986 | Ph.D. | Princeton University |
| 1984 - 1985 | Charlotte Elizabeth Procter Honorific Fellowship | Princeton University |
| 1981 | Hugh Stott Taylor Fellowship in Chemistry | Princeton University |
| 1979 - 1981 | Ph.D. Candidate | University of Washington |
| 1977 | A.B. | Harvard University |