Thomas W. Hamann
Research
Inorganic Materials / Electrochemistry
(Research Description PDF)
Hamann Group Research: The Hamann group is engaged in interdisciplinary research to address basic science issues related to new methods, molecules and materials to convert solar energy to electricity and chemical fuels. Of specific interest are regenerative and non-regenerative photoelectrochemical cells, including dyesensitized solar cells and thin-film absorber photoelectrocatalytic systems. In addition, we are interested in the use of ammonia as an energy (hydrogen) carrier and are investigating electrocatalytic nitrogen fixation and ammonia splitting.
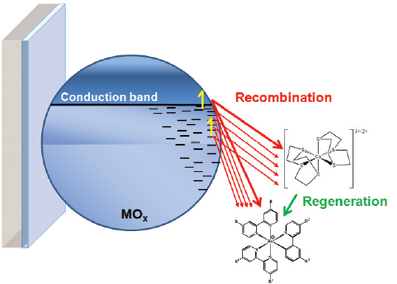
Dye Sensitized Solar Cells: Ultra-fast electron injection from a photoexcited sensitizer into a photoanode produces a charge separated state with typically high quantum efficiency. We are primarily interested in the subsequent processes of dye regeneration and recombination which control the efficiency of charge collection. We systematically vary the components involved in each reaction and interrogate them with a series of photoelectrochemical measurements. The general lessons learned will ultimately be used to develop design rules for next generation DSSCs comprised of molecules and materials which can overcome the kinetic and energetic constraints of current generation cells. In addition, we are investigating new related paradigms of solar energy conversion based on the knowledge gained from more conventional systems.
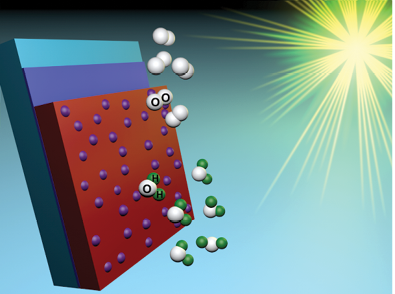
Thin Film Absorber Solar Cells: We are interested in exploring the use of thin film semiconductors to drive photoelectrochemical water splitting (solar fuel-forming) reactions. We are currently elucidating the rate limiting steps as well as water oxidation mechanism on the electrode surface. Additional topics of recent interest include understanding the effect of substrate and underlayer materials, incorporation of dopants, and surface layers (e.g. catalysts) on the water oxidation efficiency. Additional oxide, nitride and oxynitride semiconductor materials are also under current investigation.
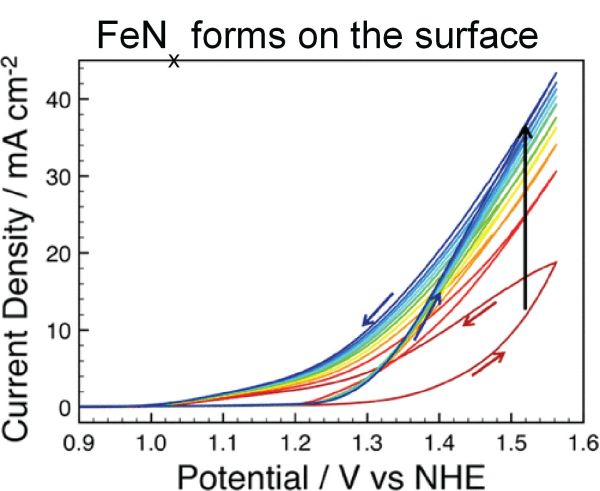
Ammonia Electrocatalysis: Nitrogen is the most abundant gas in Earth’s atmosphere and water is the most abundant liquid on Earth’s surface; combining the catalytic reduction of N2 with the oxidation of H2O to produce NH3 offers a route to scalable renewable energy storage. Liquid ammonia has an energy density comparable to methanol, and the stored chemical energy can in principle be used to generate electricity or H2 on demand. The electrolysis of liquid NH3 has received limited attention to date, however. We are also engaged on a broader collaborative effort with the Smith group to investigate the electrocatalytic conversion of liquid NH3 to H2. In addition, we are developing new electrocatalysts based on earth-abundant materials for NH3 synthesis and electrolysis.
Contact / Webpage
Area(s) of Interest
Inorganic (In)
Material (Ma)
Physical (Ph)
Selected Publications
Low-Spin Cobalt(II) Redox Shuttle by Isocyanide Coordination, Raithel, A.L., Kim, T.Y., Nielsen, K., Staples, R.J., Hamann, T.W.; Sust. Energy & Fuels 2020, 4, 2497-2507. DOI: 10.1039/D0SE00314J
Real-Time Observation of the Diffusion Mechanism Progression from Liquid to Solid-State of Transition Metal Complexes, Kim, T.Y., Wang, Y., Raithel, A.L., Hamann, T.W., ACS Energy Lett. 2020, 5, 2, 583–588.
Improved Performance Induced by in-situ Ligand Exchange Reactions of Copper Bipyridyl Redox Couples in Dye-Sensitized Solar Cells, Wang, Y., Hamann, T.W., Chem. Communic. 2018, 54, 12361–12364.
Charge-Carrier Dynamics at the CuWO4/Electrocatalyst Interface for Photoelectrochemical Water Oxidation, Shadabipour, P., Raithel, A.L., Hamann, T.W., ACS Appl. Materials & Interfaces 2020, 12, 45, 50592–50599.
Thin film photoelectrodes for solar water splitting, He, Y., Hamann, T.W., Wang, D.; Chemical Society Reviews 2019, 48, 2182–2215.
Determination of Photoelectrochemical Water Oxidation Intermediates on a-Fe2O3 Electrode Surfaces Employing Operando ATR–IR Spectroscopy, Zandi, O., Hamann, T.W.; Nature Chemistry 2016, 8, 778–783.
Recent Advances and Challenges of Electrocatalytic N2 Reduction to Ammonia, Qing, G., Ghazfar, R., Jackowski, S.T., Habibzadeh, F., Maleka Ashtiani, M., Chen, C. Smith, M.R., Hamann, T.W., Chemical Reviews 2020, 120, 12, 5437–5516.
Homogeneous Electro-Catalytic Oxidation of Ammonia to N2 Under Mild Conditions, Habib-Zadeh, F., Miller, S.L., Hamann, T.W., Smith, M.R.; Proc. Nat. Acad. Sci. 2019, 116 (8) 2849–2853.
As Precious as Platinum: Iron Nitride for Electrocatalytic Oxidation of Liquid Ammonia, Little, D.J., Edwards, D., Smith, M.R., Hamann, T.W.; ACS Appl. Mat. & Interf. 2017, 9 (19), 16228–16235.
CV
B.A., 1996, Univ. of Texas
M.S., 2000, Univ. of Massachusetts
Ph.D., 2006, California Institute of Technology
Postdoctoral Fellow, Northwestern Univ. 2006-2008
Awards
| Year | Award | Organization |
|---|---|---|
| 2015 | Kavli Fellow | |
| 2015 | SEAC Royce W. Murray Young Investigator Award | |
| 2013 | Camille Dreyfus Teacher-Scholar Award | |
| 2012 | Alfred P. Sloan Research Fellowship | |
| 2012 | National Science Foundation, CAREER award | |
| 2011 | Department of Energy, Early Career Research Program award |