Joseph Gair

Research
Selective Catalysis, Organic, Organometallic
(Research Description PDF)
The Gair group is focused on the invention and application of synthetic methods that
fine-tune structural motifs prevalent in drug discovery and catalyst design. Tools
to tailor the cores of these scaffolds will streamline access to highly valued analogs
and ultimately accelerate the discovery of new function across the molecular sciences.
Tailoring Molecules – Subtle alterations to a molecule’s structure often yield profound
changes in function. A striking illustration of this phenomenon can be seen in what
has been dubbed the “magic methyl effect”, whereby replacing a hydrogen with a methyl
group in a complex molecule can dramatically improve the potency of drugs. Given this
sensitive balance between structure and function, the discovery of new medicines relies
on access to fine-tuned analogs of lead scaffolds. The most important of these analogs
are those that preserve the functional groups of the parent molecule while subtly
adjusting the shape of the core scaffold. At present, these analogs are prepared via
lengthy synthesis from modified starting materials or entirely redesigned routes.
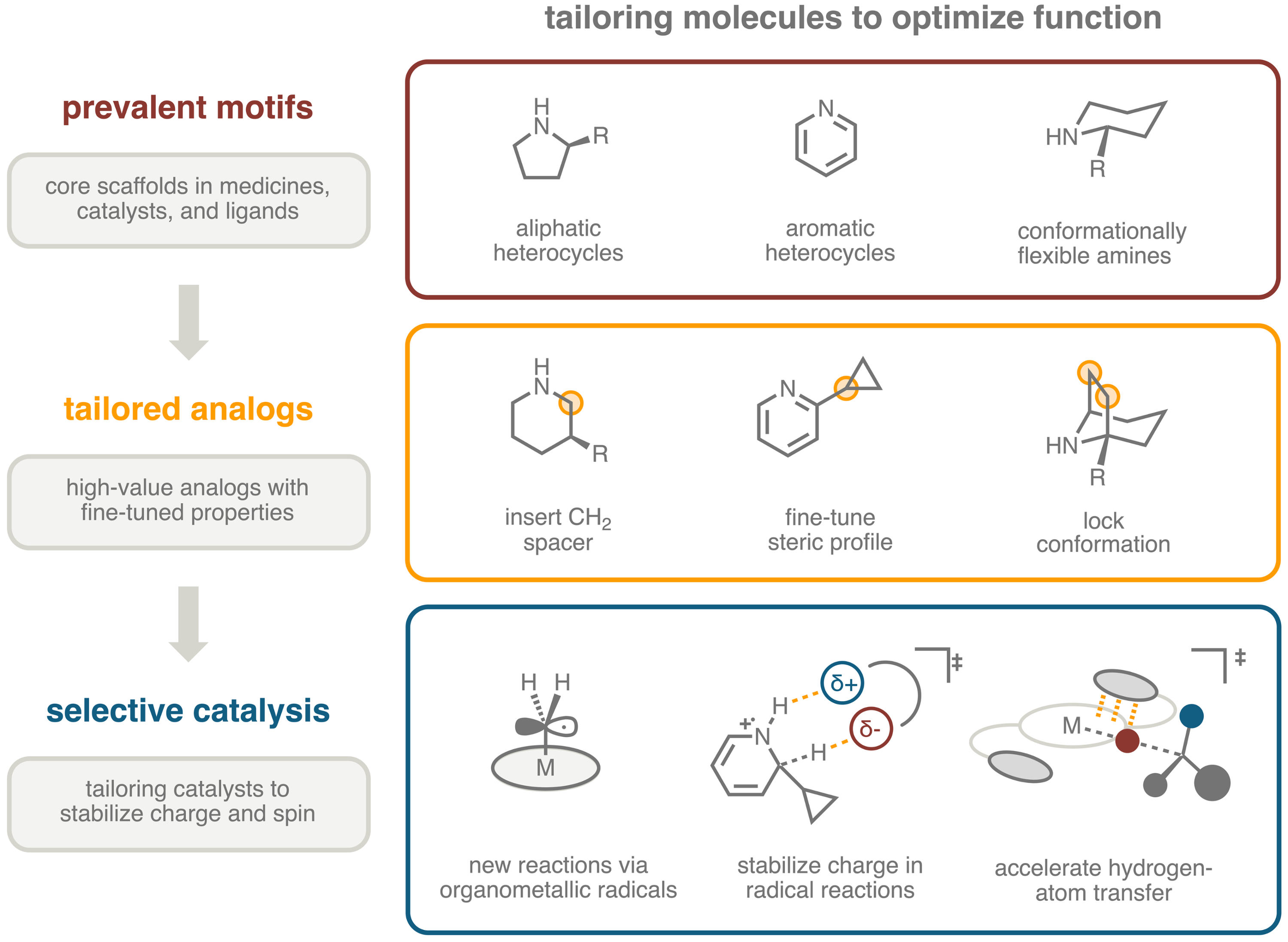
Inspired by the imagery of a tailor altering a garment to achieve a perfect fit, the Gair group aims to develop synthetic tools to tailor molecules. We are targeting transformations that fine-tune the shape of lead scaffolds without perturbing the functional groups that engender function. Our strategy is to develop new reactions that engage heterocycles and amines because these motifs are abundant across several important classes of functional molecules including medicines, biological probes, organocatalysts, and ligands. With these tools we aim to streamline access to custom-fit analogs and thereby accelerate discovery of new function across the molecular sciences.
Selective Catalysis – In addition to the practical applications outlined above, reaction development in the Gair group is centered on designing catalysts that engage in attractive interactions with transition states featuring unpaired electrons (open-shell) to achieve novel reactivity and selectivity (chemo, site, and stereo selectivity). One project area is focused on electronic tuning of organometallic radicals to achieve diverse CH2 insertion reactions with chemoselectivity dictated by the electronic structure of the catalyst. A second project area involves designing organocatalysts that complement the electrostatic topology of proton-transfer transition states to control site selectivity in radical alkylation of heteroarenes. The final project area aims to engineer hydrogen-bond acceptors on ligands of hydrogen-atom transfer catalysts to achieve enantioselective C-H functionalization. By studying the relationship between catalyst structure, rate, and selectivity in these transformations, we aim to decode the nature of attractive interactions between catalysts and open-shell transition states. Ultimately, we will apply these insights to design new catalysts for tailoring molecules.and optimization of molecular function at large.
Contact / Webpage
Area(s) of Interest
Organic (Or)
Biological (Bi)
Inorganic (In)
Organometallic (Om)
Selected Publications
Insight into the Scope and Mechanism for Transmetalation of Hydrocarbyl Ligands on Complexes Relevant to C-H Activation, Chan, N.H.; Gair J.J.; Roy, M.; Qiu, Y.; Wang, D.-S.; Durak, L.J.; Chen, L.; Filatov, A.S.; Lewis, J.C., Organometallics 2021, 40 (1), 6-10.
Palladium-Catalyzed Hydrodefluorination of Fluoroarenes, Gair, J.J.; Grey, R.L.; Giroux, S.; Brodney, M.A., Org. Synth. 2021, 98, 131-146.
Catalytic Behavior of Mono-N-Protected Amino Acid Ligands in Ligand-Accelerated C-H Activation by Palladium(II), Salazar, C.A.; Gair, J.J.; Flesch, K.N.; Guzei, I.A.; Lewis, J.C.; Stahl, S.S., Angew. Chem. Int. Ed. 2020, 59, 10873-10878.
Di-Palladium Complexes are Active Catalysts for Mono-N-Protected Amino Acid-Accelerated Enantioselective C-H Functionalization, Gair, J.J.; Haines, B. E.; Filatov, A. S.; Musaev, D.G.; Lewis, J.C., ACS Catalysis 2019, 9, 11386-11397.
Palladium Catalyzed Hydrodefluorination of Fluoro-(hetero)arenes, Gair, J.J.; Grey, R.L.; Giroux, S.; Brodney, M.A., Organic Letters 2019, 21, 2482-2487.
Synthesis, Characterization, and Theoretical Investigation of a Transition State Analogue for Proton Transfer during C-H Activation by a Rhodium-Pincer Complex, Gair, J.J.; Qiu, Y.; Khade, R.L.; Chan, N.H.; Filatov, A.S.; Zhang, Y.; Lewis, J.C., Organometallics 2019, 38 (7), 1407-1412.
Rhodium Complexes of 2,6-bis(dialkyl-phosphinomethyl)pyridines: Improved C-H Activation, Expanded Reaction Scope, and Catalytic Direct Arylation, Gair, J.J.; Qiu, Y.; Chan, N. H.; Filatov, A.S.; Lewis, J. C., Organometallics 2017, 36 (24), 4699-4706.
Mono-N-Protected Amino Acid Ligands Stabilize Dimeric Palladium(II) Complexes of Importance to C-H Functionalization, Gair, J.J.; Haines, B.E.; Filatov, A.S.; Musaev, D.G.; Lewis, J.C., Chem. Sci. 2017, 8 (8), 5746-5756.
CV
B.A., Saint John’s University
Ph.D., Univ. of Chicago
Medicinal Chemist, Vertex Pharmaceuticals
Postdoctoral Fellow, Harvard Univ. Principal Scientist 2, Merck.