Selvan Demir

Research
Single-Molecule Magnetism and Synthetic Chemistry with f-Element Compounds
(Research Description PDF)
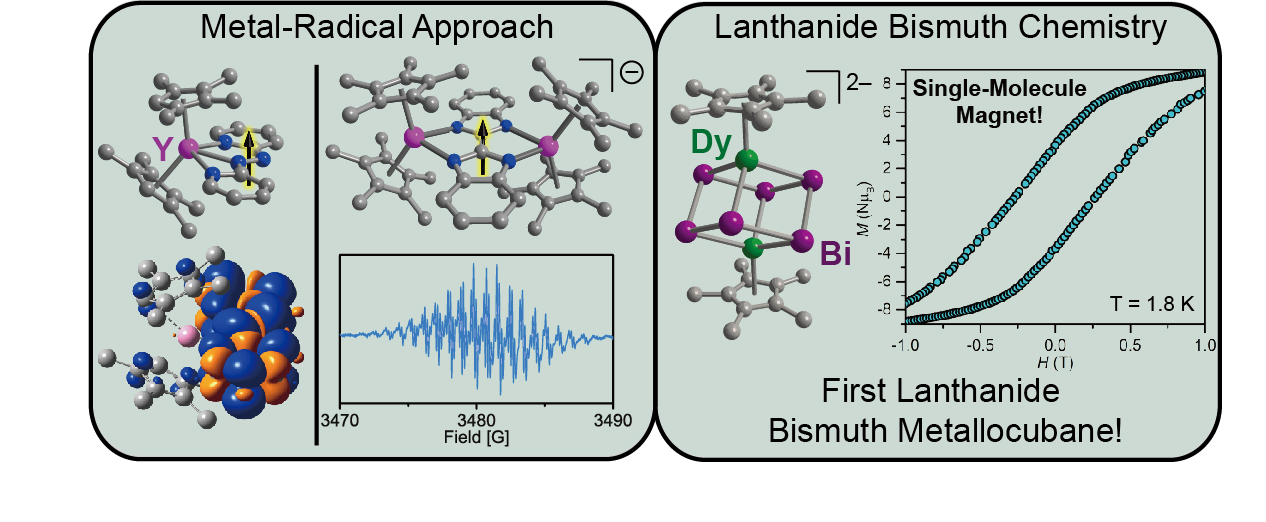
Single-molecule magnets (SMMs) are molecules that behave like nanoscopic bar magnets. They are interesting due to various potential applications in high-density information storage, molecular spintronics, and magnetic refrigeration. The challenge in realizing these applications lies in designing molecular magnets that have high operating temperatures.
The high magnetic anisotropic nature of lanthanide ions renders them particularly well-suited for the design of SMMs. This property arises from unquenched orbital angular momentum and strong spin-orbit coupling. To suppress quantum tunneling relaxation processes that lead to a shortcut of the maximum spin-relaxation barrier and thus, a weaker magnet, strong exchange coupling between the spins is required. This can be accomplished by using radical ligands with diffuse spin orbitals to penetrate the core electron density of the deeply buried 4f orbitals. We synthesize new radical ligands and investigate the spin density distributions via EPR and computational methods. Subsequently, we explore their utility for the design of radical-containing lanthanide SMMs. Another successful approach to strong coupling targets the use of heavy p-block elements since their diffuse valence orbitals facilitate better penetration of the core electron density of the lanthanide ions relative to their lighter diamagnetic analogs. In this regard, our current focus is particularly on the chemistry of bismuth, which is synthetically challenging, yet intriguing as bismuth combines with other elements in surprisingly rich ways. Quantum computers contain ‘qubits’ (short for quantum bits) as their functional units and are the next generation computers. They rely on principles of quantum information science and can execute complex algorithms which cannot be run on classical computers. A molecular approach to qubits is rare and we develop new molecular spin qubits by implementing the lanthanide ions.
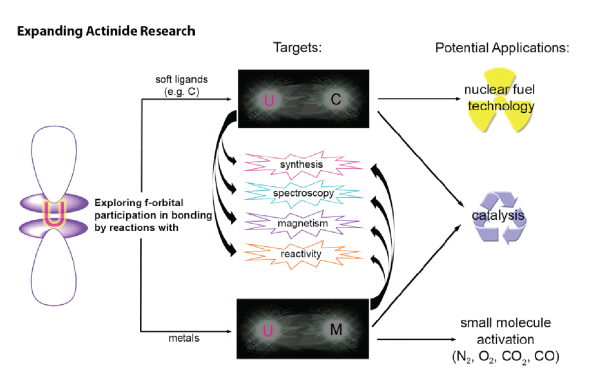
Uranium's rich chemistry lies in certain properties that results in chemical bonding and reactivity that are not seen with other elements. Uranium-ligand multiple bond compounds have been recognized as promising subjects of study for advancing uranium catalysis and actinide/lanthanide separation by shedding light on the extent of covalency in bonding of 5f orbitals. Covalency is expected to be more prominent when softer ligands are used such as C ligands rather than N or O ligands. Hence, investigating the electronic and reactivity properties of molecular uranium carbenes will improve fundamental knowledge that is crucial to the development of catalysts and new generation nuclear fuels. Besides uranium carbenes, we pursue uranium-metal bonds that are extremely rare. Such bonding moieties will uncover which 5f orbitals are involved.
Contact / Webpage
Area(s) of Interest
Inorganic (In)
Material (Ma)
Organometallic (Om)
Selected Publications
Isolation of the Elusive Bisbenzimidazole Bbim3– • Radical Anion and its Employment in a Metal Complex, Benner, F.; Demir, S., Chem. Sci. 2022, 13, 5818. Highlighted on the Inside Front Cover.
Taming Salophen in Rare Earth Metallocene Chemistry, Castellanos, E.; Benner, F.; Demir, S., Inorg. Chem. Front. 2022, 9, 1325-1336. Selected for Outside Front Cover.
Heteroleptic Rare-Earth Tris(metallocenes) Containing a Dibenzocyclooctatetraene Dianion, Pugliese, E. R.; Benner, F.; Castellanos, E.; Delano, IV, F.; Demir, S., Inorg. Chem. 2022, 61, 2444-2454. Highlighted on the Cover.
Organometallic Lanthanide Bismuth Cluster Single-Molecule Magnets, Zhang, P.; Benner, F.; Chilton, N. F.; Demir, S., Chem 2022, 8, 717–730. Highlighted on the Front Cover.
A Rare Earth Metallocene Containing a 2,2'-Azopyridyl Radical Anion, Delano IV, F.; Castellanos, E.; McCracken, J.; Demir, S., Chem. Sci. 2021, 12, 15219-15228. Highlighted on the Outside Front Cover.
Giant Coercivity and High Magnetic Blocking Temperatures for N2 3– Radical- Bridged Dilanthanide Complexes Upon Ligand Dissociation, Demir, S.; Gonzalez, M. I.; Darago, L. E.; Evans, W. J.; Long, J. R., Nat. Commun. 2017, 8, 2144.
CV
B.S., 2004, Univ. of Cologne, Germany;
M.S., 2007, Univ. of Cologne, Germany;
Ph.D., 2010, Univ. of Cologne, Germany;
Research during Ph.D. 2009-2010; University of California, Irvine;
FCI Doctoral Scholarship, 2008-2010;
Postdoctoral Fellow, 2011-2015, Univ. of California, Berkeley;
Postdoctoral Researcher, 2013-2015, Lawrence Berkeley National Laboratory;
DAAD Postdoctoral Fellowship, 2011-2013;
Junior Professor, 2016-2018, Univ. of Goettingen, Germany;
Assistant Professor, 2019-present, Michigan State University.