Babak Borhan

Research
Synthetic and Bioorganic Chemistry
(Research Description PDF)
The research interests of our lab can be subdivided into the three main areas of Bioorganic Chemistry, Synthetic Chemistry, and Organic Spectroscopy.
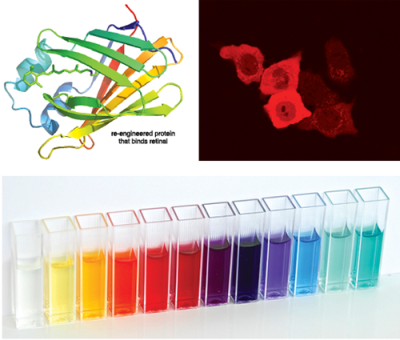 Our Bioorganic Chemistry efforts are geared towards elucidation of the interaction of bioactive compounds
with receptors and proteins. We rely heavily on de novo protein design and mimicry
of natural systems to better understand how certain biological processes occur. As
an example, we have initiated research into designing protein mimics of rhodopsin,
the protein responsible for vision, which can bind retinal as a protonated Schiff
base [PSB] (same binding mode as in rhodopsin). These protein mimics are used to investigate
the wavelength regulation mechanism that enables color vision. Currently we are exploring
the potential to use our engineered proteins as colorimetric and fluorescent proteins
for cellular tagging and intracellular pH sensors.
Our Bioorganic Chemistry efforts are geared towards elucidation of the interaction of bioactive compounds
with receptors and proteins. We rely heavily on de novo protein design and mimicry
of natural systems to better understand how certain biological processes occur. As
an example, we have initiated research into designing protein mimics of rhodopsin,
the protein responsible for vision, which can bind retinal as a protonated Schiff
base [PSB] (same binding mode as in rhodopsin). These protein mimics are used to investigate
the wavelength regulation mechanism that enables color vision. Currently we are exploring
the potential to use our engineered proteins as colorimetric and fluorescent proteins
for cellular tagging and intracellular pH sensors.
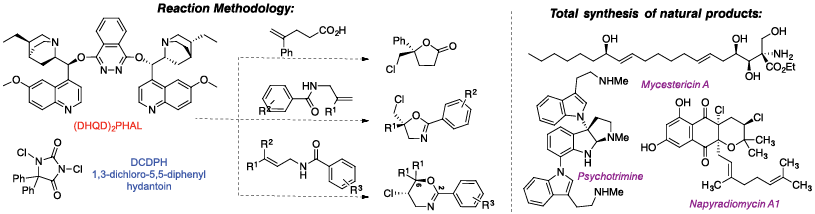
Our Synthetic Chemistry program is generally focused on the development of new reactions that utilize simple organic molecules and through designed manipulations lead to more complex systems. In most cases, our methodologies lead to the production of heterocycles with regio- and stereocontrol. These transformations are then highlighted in total syntheses of natural products that exhibit interesting biological activities. Recently we have reported the catalytic asymmetric chlorolactonization of alkenoic acids and unsaturated amides to furnish chiral heterocycles. These reactions are catalyzed by (DHQD)2PHAL in combination with various N-chlorinated hydantoins as the terminal chlorenium sources. Halofunctionalization of different compounds, understanding the mechanism of these transformations and the details of enantioselections are currently under investigation.
In the area of Organic Spectroscopy, we are interested in developing host/guest systems that can be used in the absolute
stereochemical determination of chiral compounds. 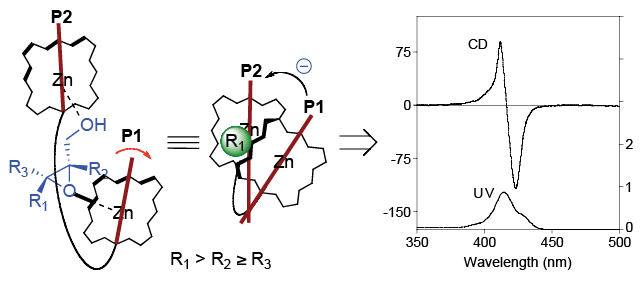 We accomplish this through the design and synthesis of chromophoric receptors, which
upon binding with the chiral compound function as reporters of chirality. We rely
heavily on Circular Dichroism (CD) as the tool for observing the host/guest interactions
between the chiral compounds and the receptors. In particular, we will take advantage
of the excitonic coupling between independently conjugated chromophores that make
up the receptors to establish non-empirical guidelines for the absolute stereochemical
determination of asymmetric centers.
We accomplish this through the design and synthesis of chromophoric receptors, which
upon binding with the chiral compound function as reporters of chirality. We rely
heavily on Circular Dichroism (CD) as the tool for observing the host/guest interactions
between the chiral compounds and the receptors. In particular, we will take advantage
of the excitonic coupling between independently conjugated chromophores that make
up the receptors to establish non-empirical guidelines for the absolute stereochemical
determination of asymmetric centers.
Contact / Webpage
Area(s) of Interest
Organic (Or)
Biological (Bi)
Selected Publications
Mimicking Microbial Rhodopsin Isomerization in a Single Crystal, Ghanbarpour, A.; Nairat, M.; Nosrati, M.; Santos, E. M.; Vasileiou, C.; Dantus, M.; Borhan, B.; Geiger, J. H., J. Am. Chem. Soc. 2019, 141(4), 1735-1741.
Cu-Catalyzed Oxidation of C2 and C3 Alkyl-Substituted Indole via Acyl Nitroso Reagents, Zhang, J.; Torabi Kohlbouni, S.; Borhan, B., Org. Lett. 2019, 21(1), 14-17.
A Near-Infrared Photoswitchable Protein- Fluorophore Tag for No-Wash Live Cell Imaging, Sheng, W.; Nick, S. T.; Santos, E. M.; Ding, X.; Zhang, J.; Vasileiou, C.; Geiger, J. H.; Borhan, B., Angew. Chem. Int. Ed. Engl. 2018, 57, 14742-14746.
Absolute and relative facial selectivities in organocatalytic asymmetric chlorocyclization reactions, Marzijarani Salehi, N.; Yousefi, R.; Jaganathan, A.; Ashtekar, K. D.; Jackson, J. E.; Borhan, B., Chem. Sci. 2018, 9, 2898-2908.
Highly Regio- and Enantioselective Vicinal Dihalogenation of Allyl Amides, Soltanzadeh, B.; Jaganathan, A.; Yi, Y.; Yi, H.; Staples, R. J.; Borhan, B., J. Am. Chem. Soc. 2017, 139(6), 2132-2135.
Absolute Stereochemical Determination of Asymmetric Sulfoxides via Central to Axial Induction of Chirality, Gholami, H.; Zhang, J.; Anyika, M.; Borhan, B., Org. Let. 2017, 19, 1722-1725.
Nucleophile-Assisted Alkene Activation: Olefins Alone are Often Incompetent, Ashtekar, K.D.; Vetticatt, M.; Yousefi, R.; Jackson, J. E.; Borhan, B., J. Am. Chem. Soc. 2016, 138(26), 8114-8119.
Sensing Remote Chirality: Stereochemical Determination of beta-, gamma-, and delta-Chiral Carboxylic Acids, Tanasova, M.; Anyika, M.; Borhan, B., Angew. Chem. Int. Ed. Engl. 2015, 54, 4274-4278.
Tuning the Electronic Absorption of Protein-Embedded All-trans-Retinal, Wang, W.; Nossoni, Z.; Berbasova, T.; Watson, C. T.; Yapici, I.; Lee, K. S. S.; Vasileiou, C.; Geiger, J. H.; Borhan, B., Science 2012, 338, 1340-1343.
CV
B.S., 1988, Univ. of California, Davis
Ph.D., 1995, Univ. of California, Davis
Postdoctoral Fellow, 1995-1998, Columbia Univ.
Awards
| Year | Award | Organization |
|---|---|---|
| 2017 | Outstanding Faculty Award | College of Natural Science |
| 2015 | Meritorious Faculty Award | College of Natual Science |
| 2004 | Teacher-Scholar Award | College of Natural Science |
| 2003 | Teacher-Scholar Award | College of Natural Science |
| 2002 | Teacher-Scholar Award | College of Natural Science |
| 2002 | University Teacher-Scholar Award | Michigan State University |
| 1995 - 1998 | Postdoctoral Fellow | Columbia University |
| 1995 | Ph.D. | University of California, Davis |
| 1994 | Outstanding Teaching Assistantship Award | University of California, Davis |
| 1990 - 1993 | Full foreign tuition fellowship | University of California, Davis |
| 1989 | Bradford Borges Graduate Scholarship | |
| 1988 | Bachelor of Science | University of California, Davis |
| 1987 - 1988 | University of California Regents Scholarship | University of California, Davis |
| 1985 - 1986 | University of California Undergraduate Scholarship | University of California, Davis |
| Member | Sigma Xi Honor Society (Michigan State University) |