Gary J. Blanchard

Research
Synthesis and Spectroscopy of Interfaces
(Research Description PDF)
The ability to control the physical properties and chemical selectivity of an interface is an issue central to areas of science including cellular function, energy storage, heterogeneous catalysis and chemical sensing. The Blanchard group works on the design, synthesis and characterization of interfaces with an eye toward achieving this control. We are currently focusing our energies on catalytic and ordered systems because of their broad utility.
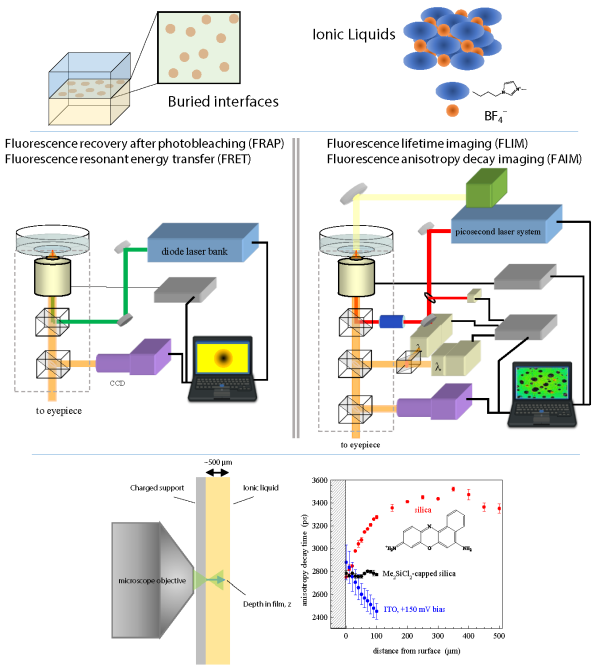
Long Range Order in Ionic Liquids. Ionic liquids are a class of materials that can be described as salts that are liquids at room temperature. These materials are typically viscous fluids and they have found use in areas ranging from organic synthesis to chemical sensing and energy storage. The organization that exists within ionic liquids is not well understood and has until recently been thought to be on the orders of nanometers in length. Our recent work has shown that when ionic liquids are placed in proximity to a charged surface, the charge induces order that persists on the sub-millimeter lengthscale — five orders of magnitude greater than expected. These new findings not only provide insight into the structure of these systems, but also open the door to novel applications in energy storage and electronically-controlled optics.
Characterizing interfacial heterogeneity. We quantitate molecular motion on molecular length scales and over micron to millimeter length scales, using two complementary microscopy techniques. Using these techniques, we can evaluate the fluidity of a wide range of interfaces and, significantly, we can now characterize transient structural non-uniformities in mono- and bilayer films. This latter capability offers a new way to explore the presence of previously invisible spatial variations in chemical composition, with applications ranging from sensor interface design to in situ plasma membrane characterization.
Controlling interfacial fluidity. Covalently bound interfacial adlayers are not fluid, and fluid adlayers are not physically or chemically robust. These limiting cases have frustrated advances in fields such as molecular-scale lubrication, chemical separations and cellular adhesion. We are developing a novel family of interfaces that can be bound to an interface and at the same time retain the properties of a fluid. Both the thermodynamic driving force for complexation and the kinetics of surface diffusion can be controlled through metal ion complexation, system pH, the surface complexing moieties, and the amphiphile head groups.
Measuring fluidity in live cell plasma membranes. Diabetic retinopathy is a disease state that can compromise the vision of diabetic patients. It’s origins are thought to be related to chronic exposure to high glucose conditions. Diabetic hyperglycemia leads to changes in the plasma membranes of circulating angiogenic cells which make them less rigid and thus less available to perform their repair functions in vivo. We work with Professor Julia Busik’s group (physiology) to evaluate the fluidity of live cell plasma membranes using rotational and translational diffusion measurements of fluorescent molecules introduced to the cells. This work is providing new insight into the molecularscale consequences of diabetic disease state on cells.
Contact / Webpage
Area(s) of Interest
Analytical (An)
Biological (Bi)
Material (Ma)
Selected Publications
Effects of Ethanol and n-Butanol on the Fluidity of Supported Lipid Bilayers, Masroor Hossain and G. J. Blanchard, Chem. Phys. Lipids 2021, 238, 105091, 1-7.
Ceramide-Mediation of Diffusion in Supported Lipid Bilayers, Masroor Hossain and G. J. Blanchard, Chem. Phys. Lipids 2021, 238, 105090, 1-7.
Spectroscopic Analysis of Cu(II)- Complexed Thin Films to Characterize Molecular-Level Interactions and Film Behavior, B.A. Capistran and G.J. Blanchard, Langmuir 2021, 37, 5089-5097.
Metal Ion Dependent Interfacial Organization and Dynamics of Metal Phosphonate Monolayers, C. Livingston and G.J. Blanchard, Langmuir 2021, 37, 4658-4665.
Charge-Induced Birefringence in a Room Temperature Ionic Liquid, Yufeng Wang, G.M. Swain and G.J. Blanchard, J. Phys. Chem. B 2021, 125, 950-955.
Local and Long Range Organization in Room Temperature Ionic Liquids, Y. Wang, F. Parvis, Md. I. Hossain, K. Ma, R. Jarosova, G.M. Swain and G.J. Blanchard, Langmuir 2021, 37, 605-615. Invited Feature Article.
Isoenergetic Two-Photon Excitation Enhances Solvent-to-Solute Excited-State Proton Transfer, J. Lahiri, M. Moemeni, J. Kline, I. Magoulas, S.H. Yuwono, M. Laboe, J. Shen, B. Borhan,* P. Piecuch,* J.E. Jackson,* G.J. Blanchard,* and M. Dantus,* J. Chem. Phys. 2020, 153, 224301, 1-14.
Steric Effects in Light-Induced Solvent Proton Abstraction, J. Lahiri, M. Moemeni, J. Kline, B. Borhan, I. Magoulas, S.H. Yuwono, P. Piecuch, J.E. Jackson, G.J. Blanchard and M. Dantus, Phys. Chem. Chem. Phys. 2020, 22, 19613-19622.
Selective LXR agonist, DMHCA, corrects the Retina-bone Marrow Axis in Type 2 Diabetes, P. Cristiano, S.F. Vieira, A.L. Longhini, T. Lydic, B. Asare-Bediako, S.S Hammer, M. Hossain, D. Crossman, M.S. Sielski, Y. Adu-Agyeiwaah, M. Dupont, J.L. Floyd, S. Li Calzi, G.J. Blanchard, J. Busik and M.B. Grant, J. Clin. Invest. 2020, 5, 137320, 1-21.
CV
B.S., 1981, Bates College
Ph.D., 1985, Univ. of Wisconsin-Madison
Member of Technical Staff, 1985-91, Bell Communications Research
Awards
| Year | Award | Organization |
|---|---|---|
| 2020 | ANACHEM Award | |
| 2017 | Outstanding Faculty Award | College of Natural Science |
| 2011 | Gold Medal Award from the New York Section of the Society for Applied Spectroscopy | |
| 2004 | Member | International Conference on Electode Processes (Scientific Committee) |
| 2004 | Member | National Institutes of Health (NIH Repairative Medicine Study Section) |
| 2001 | Member | NASA (NASA Crystal Growth Review Panel) |
| 2001 - 2002 | Member | American Chemical Society (ACS Award in Chemical Instrumentation Jury) |
| 2001 | Discussion Leader | Gordon Research Conference (Analytical Chemistry) |
| 1999 | Member | National Science Foundation (NSF-CCLI Review Panel) |
| 1999 | Organizer | American Chemical Society (Symposia on Polymer Characterization and Interface Characterization, Fall National Meeting) |
| 1998 | NSF pecial Creativity Extension | National Science Foundation |
| 1998 | Member | NASA (Environmental Health Review Panel) |
| 1998 | Special Creativity Award | National Science Foundation |
| 1997 | Organizer | FACSS (Molecular Spectroscopy, Materials and Interfaces Program) |
| 1997 | Member | Optical Society of America (International Organizing Committee, Optical Society of America Fall Meeting) |
| 1996 | Organizer | National Science Foundation (Keystone Conference on Reconnecting the Academic and Industrial Analytical Communities - GOALI Workshop) |
| 1995 - 1996 | Member | National Science Foundation (NSF SBIR Review Panels) |
| 1995 - 1996 | Member | (Findeis Award Jury) |
| 1985 | Ph.D. | University of Wisconsin-Madison |
| 1983 | DuPont Departmental Fellowship | |
| 1981 | Bachelor of Science | Bates College, Lewiston, ME |
| 1981 | Graduate with Honors (Chemistry) | Bates College, Lewiston, ME |
| 1980 | Eastern Analytical Symposium Student Award | |
| 1980 | Summer Internship | American Chemical Society (Analytical Division) |