John Frost

Research
Green Chemistry
Green chemistry is being elaborated that enables CO2 fixed by plants to be converted into chemicals currently derived from the BTX (benzene toluene xylene) fraction of petroleum refining. Nonrenewable fossil fuel feedstocks, carcinogenic starting materials and toxic intermediates are avoided. In addition, an array of new monomers is being synthesized to identify structures that are: (a) free of endocrine disruption activity, and (b) lead to polymers and plasticizers characterized by novel materials properties.
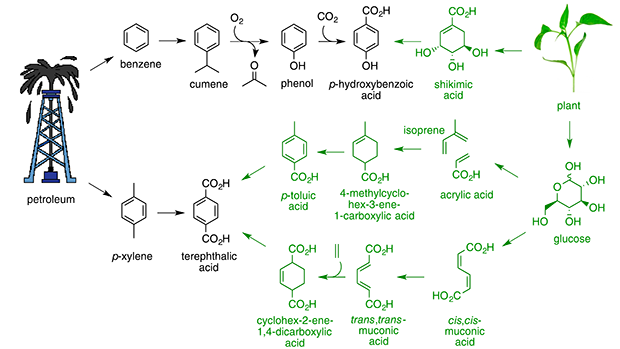
Current commercial synthesis of p-hydroxybenzoic acid begins with BTX-derived benzene and proceeds through cumene and phenol as intermediates. Carboxylation of potassium phenolate affords p-hydroxybenzoic acid monomer, which typically constitutes 50% of the mass of liquid crystalline polymers. A green synthetic alternative has been elaborated whereby p-hydroxybenzoic acid is synthesized in a single step in high conversion and good selectivity from nontoxic shikimic acid. Shikimic acid, in turn, is microbially synthesized from plant-derived glucose or isolated directly from plants such as Ginkgo biloba. Shikimic acid’s solubility in n-butanol and propensity to crystallize from n-butanol facilitate its isolation from fermentation broth or plant tissue. Green synthesis of p-hydroxybenzoic acid eliminates the need for using carcinogenic benzene as a starting material and toxic phenol as an intermediate.
BTX-derived xylene is industrially oxidized to terephthalic acid, which is polymerized with ethylene glycol to produce poly(ethylene terephthalate) PET. Over 50 × 109 kg of terephthalic acid are globally produced each year. Two green synthetic alternative routes have been developed. Isoprene and acrylic acid microbially synthesized from glucose undergo a cycloaddition to form 4-methylcyclohex-3-ene-1-carboxylic acid. Dehydrogenation affords terephthalic acid. Alternatively, cis,cis-muconic acid microbially synthesized from glucose is isomerized and the resulting trans,trans-muconic acid reacted in a cycloaddition with bioethanol-derived ethene to yield cyclohex-2-ene-1,4-dicarboxylic acid. Dehydrogenation affords terephthalic acid. In addition to use of renewable feedstocks, the new routes enable the first practical synthesis of substituted terephthalates when substituted acrylic acids and substituted ethenes are employed. Furthermore, a parallel world of 1,4-cyclohexane and 1,4-cyclohexene 1,4- dicarboxylic acids has been created, which affords unique opportunities to avoid aromatic-associated, endocrine disruption activity while enabling the fabrication of novel materials.
Contact
Area(s) of Interest
Organic (Or)
Biological (Bi)
Selected Publications
B-O-B catalyzed cycloadditions of acrylic acids, Zhang, P.; Kriegel, R. M.; Frost, J. W. ACS Sustainable Chem. Eng. 2016, 4, 6991-6995.
Synthesis of Terephthalic Acid from Methane, Zhang, P.; Nguyen, V.; Frost, J. W., ACS Sustainable Chem. Eng. 2016, 4, 5998-6001.
Synthesis of Biobased Terephthalic Acid from Cycloaddition of Isoprene with Acrylic Acid, K.K. Miller, P. Zhang, Y. Nishizawa- Brennen, and J. W. Frost, ACS Sustainable Chem. Eng. 2014, 2, 2053−2056.
CV
B.S., 1977, Purdue Univ.
Ph.D., 1981, Massachusetts Institute of Technology.
Awards/Honors
| Year | Award | Organization |
|---|---|---|
| 2010 - 2010 | Science and Technology Awards from Corp! magazine | Draths Corporation |
| 2002 | Organizer | U.S. Department of Energy (DOE) Catalysis Workshop - biocatalysis working group and authored sections dealing with biocatalysis in the report entitled "Opportunities for Catalysis Science in the 21st Century" |
| 2000 | Distinguished Faculty Award | Michigan State University (College of Natural Science) |
| 2000 | Outstanding Faculty Award | College of Natural Science |
| 1999 | Participant | EPA/NSF (Joint Technology for a Sustainable Environment Review Workshop) |
| 1999 | Session Organizer | "Industrial Chemicals" 21st Symposium on Biotechnology for Fuels and Chemicals |
| 1999 | Co-Chairperson | National Science Foundation/DOE (Workshop on Molecular Energy and Environmental Science) |
| 1998 | The Presidential Green Chemistry Challenge Award | Environmental Protection Agency |
| 1995 | Participant | National Institutes of Health (Metabolic Engineering Workshop - this gathering advised NIH on the establishment of an initiative in metabolic engineering.) |
| 1994 | Facilitator | U.S. Environmental Protection Agency and the National Science Foundation "Workshop on Green Syntheses and Processing in Chemical Manufacturing" benign organic synthesis working group |
| 1994 | Organizing Committee Member | Council for Chemical Research Workshop on Environmental Chemistry |
| 1992 | Co-Chairperson | "Biocatalysis"Gordon Research Conference |
| 1992 | Co-organizer | "Workshop in Environmental Chemistry" National Science Foundation |
| 1991 | Cyanamid Faculty Award | |
| 1988 | Co-organizer | "Strategies and Opportunities at the Interface Between Chemistry, Chemical Engineering and Life Sciences" The 39th Industrial Affiliates Symposium, Stanford University |
| 1987 - 1992 | Teacher-Scholar Award | Camille and Henry Dreyfus Foundation |
| 1987 - 1989 | Lilly Grantee | |
| 1987 | Organizer | National Science Foundation Workshop |
| 1987 - 1989 | Alfred P. Sloan Fellowship | Alfred P. Sloan Foundation |
| 1985 | Summer Faculty Fellowship | Rohm and Haas |
| 1985 | Searle Scholar |