Swain Research Group
We thank you for your interest in our research group at Michigan State University. We encourage you to explore this website to learn more about our interdisciplinary research and the students who are engaged in the projects.
Google Scholar: http://scholar.google.com/citations?user=WI4v_OoAAAAJ&hl=en
ResearcherID: B-302302010 http://www.researcherid.com/rid/B-3023-2010
ORCID: http://orcid.org/0000-0001-6498-8351
Current MSU Affiliations:
Professor - Department of Chemistry (analytical)
Director of the RECR Education Program, Office of Research and Innovation (2023-present)
RECR Education Coordinator, The Graduate School (2019-present)
Director, NSF REU Site: Cross-Disciplinary Research in Sustainable Chemistry and Chemical Processes (2014 - 2025)
MSU Fraunhofer - Center for Coatings and Diamond Technologies
International Affiliations:
Visiting Guest Professor, Gdansk University of Technology (Poland), 2025
Special Visiting Professor, Keio University (Japan), 2023
Japan Society for the Promotion of Science Short-Term Research Fellow (2020-2021)
Fulbright Specialist - Czech Republic (2018)
Visiting Professor, Domaine Universitaire, Grenoble, France (summer 2001)
Special Visiting Researcher (CAPES), Universidade Federal de São Carlos (Brazil), 2013-2016
Service:
Academic Advancement Network Leadership Fellow, MSU (2018-2019)
Member MSU Steering Committee (2017-2020)
At-Large Member MSU Faculty Senate (2017-2020)
At-Large Member MSU University Council (2017-2020)
ACS Committee on Professional Training, Member (2015-2020)
Editor-in-Chief, Elecroanalysis (Wiley), 2021-present
Editor, Electroanalysis, 2019-2021
Associate Editor, Critical Reviews in Analytical Chemistry, 2014-present
Advisory Board, Advanced Engineering Materials, 2014-present
Editor-in-Chief, Diamond and Related Materials (Elsevier), 2011-2014
Editor, Diamond and Related Materials, 2009-2011
Editorial Board, Diamond and Related Materials (Elsevier), 2006-2015
Research in our group is highly interdisciplinary and collaborative. Carbon material science is at the core of most of the work. We study electrochemical reaction kinetics, mechanisms, and interfacial capacitance at various types of carbon electrodes, including boron-doped diamond (BDD) and nitrogen-incorporated tetrahedral amorphous carbon (ta-C:N), before and after chemical modification. The research utilizes planar thin films, optically transparent electrodes, microelectrodes, and powderous forms of BDD and ta-C:N. Screen-printed and inkjet-printed carbon electrodes are also utilized. A goal is to tailor the properties of these carbon electrodes for application in (i) the electrosynthesis of value-added products from sustainably-derived source materials and pollutants, and (ii) electrochemical sensing, biosensing and immunosensing assays for biomarkers of respiratory disease in exhaled breath condensate. We also study how chemical modification of carbon surfaces affect electron-transfer kinetics for redox systems in aqueous and organic solvent/electrolyte systems and in room temperature ionic liquids. Finally, research is being conducted to understand how different surface pretreatments and coating systems affect the corrosion resistance and mechanical properties of metal alloys prepared by traditional methods and by addidive manufacturing (3D printing).
A variety of analytical tools are employed in our research including electrochemical and spectroelectrochemical methods of analysis, electron and optical microscopy, atomic force microscopy, optical profilometry, x-ray diffraction, x-ray photoelectron spectroscopy, and Raman spectroscopy.
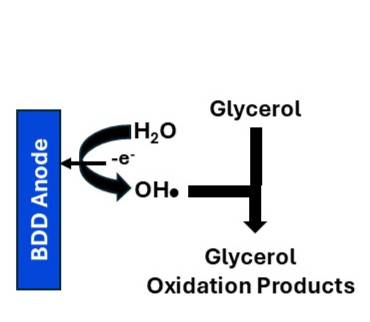
1. Electrosynthesis with BDD Electrodes to Produce Value-Added Products. Boron-doped diamond (BDD) thin-film electrodes are next-generation sp3-bonded carbon materials. They exhibit excellent electrochemical properties, have high chemical stability, and are inexpensive and available commercially making them suitable for sensing, water treatment, energy conversion, and electrosynthesis. Electrosynthesis is a process in which electrical energy is used to drive oxidation or reduction reactions enabling the efficient and sustainable production of value-added products. Electrosynthesis can replace more traditional and polluting processes and can be conducted under mild conditions. BDD electrodes have two properties critical for electrosynthesis: (i) dimensional stability and degradation resistance and (ii) a wide working potential window in aqueous electrolyte solutions; a property that enables electrochemical reactions to be performed over a wider potential range than is possible with other electrodes (i.e., less interference by deleterious solvent-electrolyte electrolysis). In this project, fundamental research will be conducted to demonstrate that BDD electrodes can function efficiently and stably for the electrooxidation of glycerol to produce value-added products. Glycerol is primarily produced as an abundant, low-cost by-product of biodiesel manufacturing. Due to a global surplus, there is a technological need to develop a sustainable method to convert crude glycerol into a variety of high-value chemicals.
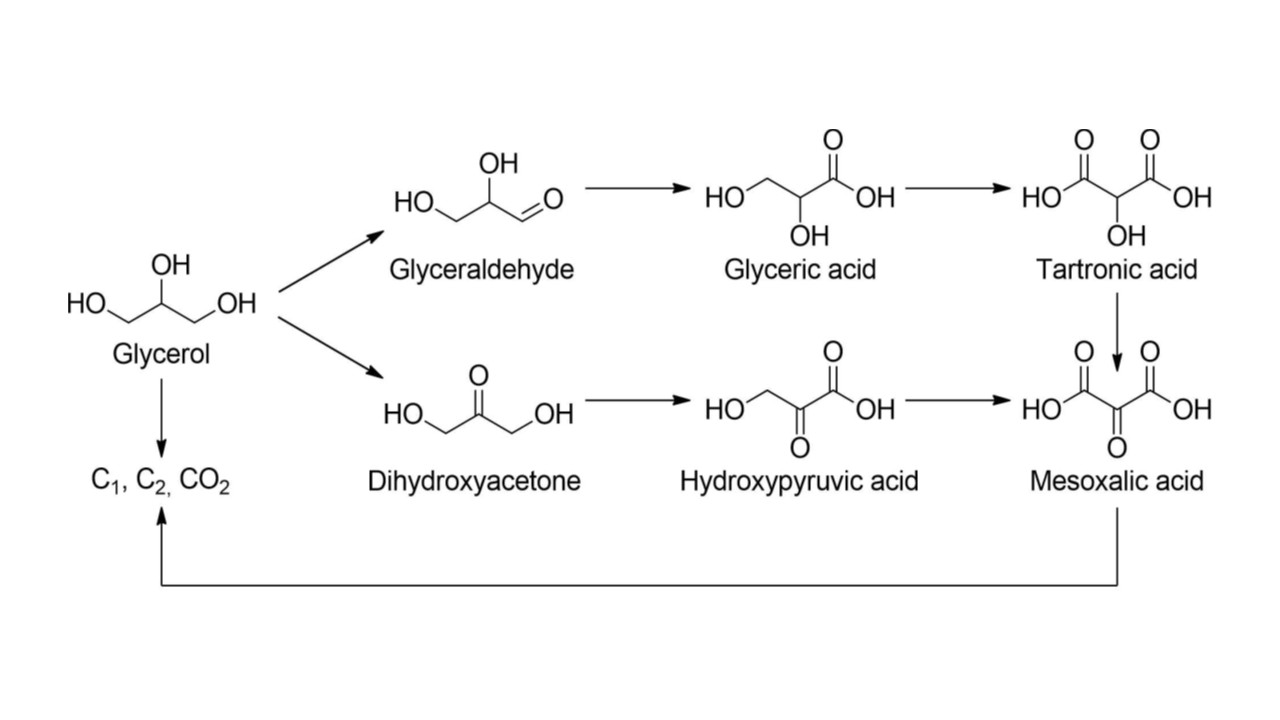
The electrooxidation of glycerol can yield C1, C2, and C3 organic compounds, with product selectivity that depends on the BDD electrode material properties and reaction conditions (potential, electrolyte composition and pH). A range of value-added products can be produced, including dihydroxyacetone (DHA), glyceric acid (GLA), formic acid (FA), lactic acid, glycolaldehyde, and oxalic acid. These products have value in diverse applications across multiple industries. Research is needed to understand how the electrode microstructure, boron-doping level, surface chemistry, applied potential and current density, solution conditions (aqueous electrolyte and pH), temperature, and flow affect the oxidation of biomass-derived glycerol to form different products.
2. Electrochemical Diagnostics for Human and Animal Health Care. Students will work on first-generation, point-of-care diagnostic technology useful for the clinical monitoring, care delivery, and disease management of human subjects with lung disease. Additionally, we are working on the technology for application in the management of bovine respiratory disease (BRD) in cattle.
One project aim is to establish the clinical value of exhaled breath condensate (EBC) as a transformative, non-invasive liquid biopsy specimen for lung cancer disease management. Building on this innovative platform, we are working to prototype two first-in-class electrochemical immunosensing assays designed for longitudinal, point-of-care monitoring of critical biomarkers.
The first sensing assay targets soluble PD-L1 (programmed death ligand 1), an immune checkpoint protein expressed by tumor cells that is a validated predictor of response to immune checkpoint inhibitor therapy, particularly in non–small cell lung cancer (NSCLC). By enabling serial PD-L1 measurements in real time, this technology has the potential to overcome current limitations of tissue-based assays, offering a dynamic and personalized approach to immunotherapy management.
The second assay measures BNP (β-type natriuretic peptide), a sensitive biomarker of early cardiac injury and a strong predictor of chemotherapy-induced cardiotoxicity. Early identification of subclinical cardiac damage could allow timely intervention, reduce treatment-related morbidity and enable safer delivery of life-prolonging therapies.
If successful, this work will deliver novel diagnostic tools that integrate seamlessly into clinical workflows, advancing precision oncology and survivorship care. Ultimately, these innovations have the potential to redefine biomarker monitoring by shifting from invasive, static assessments to non-invasive, dynamic, and patient-friendly diagnostics, thereby improving outcomes for patients with lung cancer.
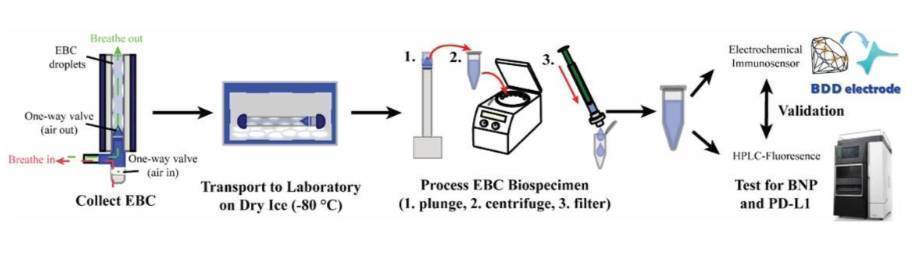
The above figure shows the workflow for the EBC collection, processing and electrochemical analysis. The EBC biospecimens are collected from a patient during normal breathing for 3-5 min, which produces 1-2 mL.
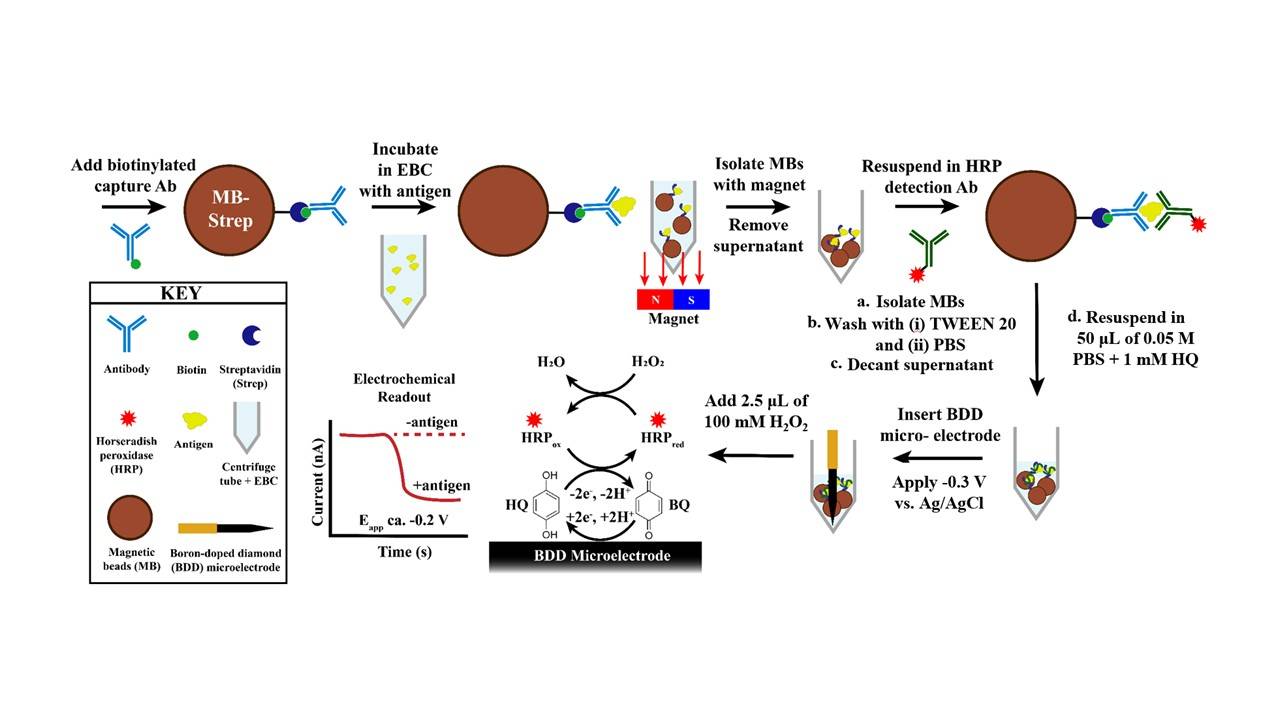
The above figure shows the conventional sandwich-style electrochemical immunosensing assay for PD-L1 and BNP. The difference is the capture and detection antibodies used for each biomarker.
Similar work is ongoing with a focus of measuring biomarkers of bovine respiratory disease (BRD) in cattle.
Both projects are collaborative with physicians in the College of Medicine and clinicians in the College of Veterinary Medicine.
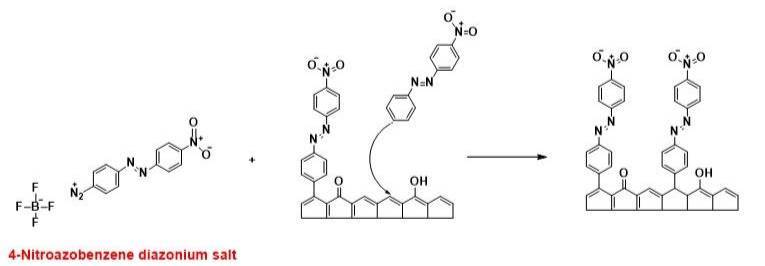
3. Chemically Modified Carbon Surfaces-Impacts on Electrochemical Reaction Kinetics. The surface chemistry of carbon materials, such as diamond, glassy carbon, carbon nanotubes and graphene, and metals (Au, Pt, Ni) can be controlled by bonding organic layers, such as through the use of aryl diazonium salts. Reductive electron transfer to the diazonium molecule in solution results in the homolytic cleavage of dinitrogen and the generation of an aryl radical. The radical formed at the electrode-solution interface subsequently bonds to the surface through carbon-carbon and metal-carbon bonds. Aryl diazonium adlayers can be formed in aqueous and non-aqueous solution using electrochemically-assisted and spontaneous formation processes. The spontaneous formation involves simply immersing the substrate in a diazonium solution under open circuit conditions for a fixed time. Current research is focused on the spontaneous formation process. We seek to understand how the diazonium molecule type, solution medium (aqueous vs. non-aqueous), immersion time, and concentration affect the adlayer coverage, thickness, chemistry and blocking properties for redox reactions. The figure below shows the general formation process of an aryl diazonium adlayer, in this example, 4-nitrophenyl diazonium (4-NAB).
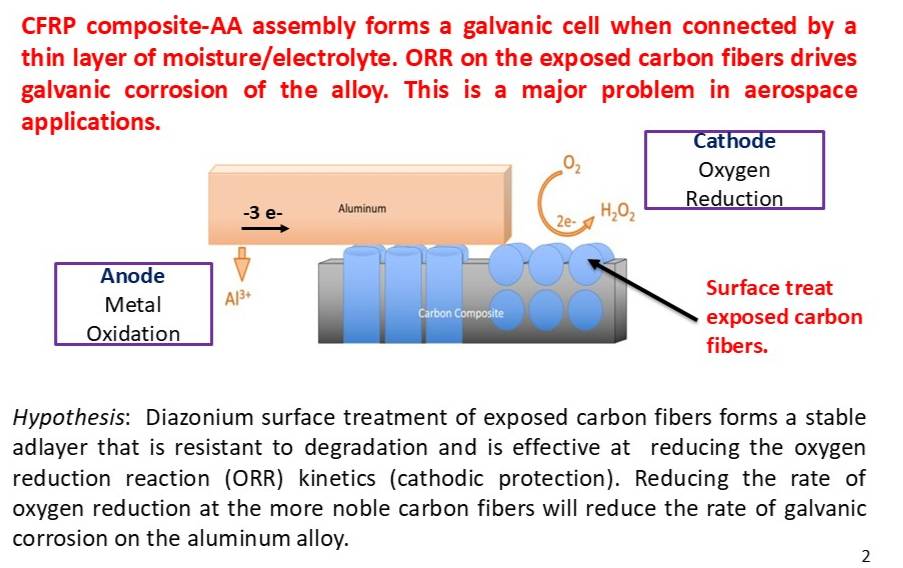
The oxygen reduction reaction (ORR) is a redox reaction of interest. In aerospace applications, carbon fiber composites are used along with aluminum alloys. The locations where these two dissimilar materials are joined can lead to galvanic corrosion of the aluminum alloy. At the composite-alloy joints, a galvanic cell will form when the two are electrically connected a thin layer of moisture/electrolyte. The carbon fibers of the composite are more noble than the aluminum alloy. As such, the oxygen will be reduced at the carbon fibers (cathode) and the aluminum alloy (anode) will undergo oxidation. The oxidation or corrosion of the alloy will occur at a higher rate than it otherwise would standalone. This galvanic corrosion is a major problem in aerospace applications. The diazonium adlayer provides cathodic inibition and thus reduces the rate of galvanic corrosion on the nearby alloy. The concept is shown in the figure below.
Electrochemical methods, various microscopies and surface science tools are being used for material and surface characterization.
4. Surface Treatments and Coating Systems for Corrosion Control on Metal Alloys. In this project, research is being conducted to better understand how different surface treatments (heat treatment and ultrasonic vibration) and coating systems (oxides and conversion coatings) affect the corrosion resistance of metal alloys. The surface treatments can alter morphology, microstructure and surface chemsitry, which will impact the aqueous corrosion resistance. The coating systems are designed to limit contact of the environment with the underlying metal. How these coatings form, their chemical composition, adhesion and defect density depend on the surface morphology, microstructure and chemical composition. This work is being performed using traditionally prepared aluminum and titanium alloys as well as with additive manufactured (AM), or 3D printed metal alloys. AM is the process of fabricating objects layer-by-layer, as opposed to traditional subtractive manufacturing technologies. We seek to understand how the surface texture, alloy microstructure, and elemental composition affect the electrochemical behavior of AM aluminum, steel, and titanium alloys prepared by selective laser melting. Electrochemical methods, various microscopies and surface science tools are being used for material and surface characterization. This project is collaborative with colleagues in the Department of Chemical Engineering and Material Science, and the Fraunhofer USA Center Midwest (CMW).
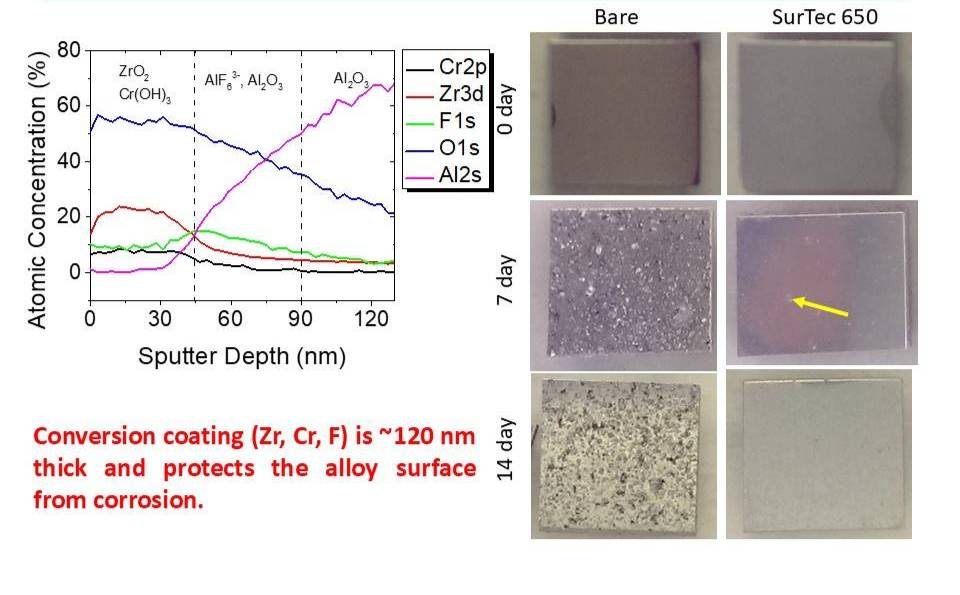
The figure below shows some characterization data for a trivalent chromium process (TCP) conversion coating (~150 nm thick) applied to an aluminum alloy surface and the corrosion protection it provides. On the left are XPS depth profile data for the TCP conversion coating elements, Zr, Cr and F. The coating is zirconia/hydroxyzirconia with added chroimium hydroxide. The chromium hydroxide is enriched on the outer part of the coating. The optical micrographs on the right clearly show the corrosion protection afforded by the TCP (SurTec 650) coating during a 14-day neutral salt spray exposure.
.JPG)