Organoborane Chemistry
Organoboranes perform beautifully in Pd-mediated cross-coupling reactions also know as Suzuki couplings. Indeed, the Suzuki cross-coupling is the third most common C–C bond forming reaction used for the preparation of drug candidates.
Over 20 years ago, we began a collaboration with Professor Milton R. “Mitch” Smith, III to explore catalytic aromatic C-H activation-borylation as a new approach to novel and highly useful aromatic building blocks for organic synthesis. In 1999, Professor Smith and his co-workers first reported on Ir-based catalysts that were remarkably selective for the C-H activation-borylation of unactivated arenes.
The mildness of these catalysts and reaction conditions under which they operate allow the borylation of arenes containing a variety of functional groups. Moreover the regiochemical outcomes of these reactions often complement those observed in traditional aromatic substitutions. Owing to these features, catalytic aromatic C-H activation-borylation represents a new and attractive approach to aromatic and heteroaromatic boronic esters. Furthermore, we have designed reaction sequences that merge C–H activation-borylation reactions with cross-couplings, C–N bond forming reactions, oxidations, reductions, deuterium/boron exchange reactions, etc. These processes allow unique access to functionalized aromatic building blocks that are novel or only accessible by protracted, costly, and otherwise unattractive routes.
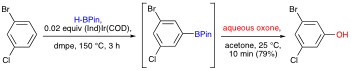
An example of our one-pot C–H activation/borylation/oxidation based preparation of phenols is shown below.
Selected organoborane related publications:
“Balancing Reactivity, Regioselectivity and Product Stability in Ir-catalyzed Ortho C–H Borylations of Anilines by Modulating the Diboron Partner” Montero Bastidas, J. R.; Yadav, A.; Lee, S.; Ghaffari, B.; Smith, M. R., III; Maleczka, R. E., Jr. Org. Lett. 2024, 26, 5420–5424.
“A Hydrazone Ligand for Iridium-Catalyzed C–H Borylation: Enhanced Reactivity and Selectivity for Fluorinated Arenes” Peruzzi, C. D.; Miller, S. L.; Dannatt, J. E.; Maleczka, R. E., Jr.; Smith, M. R., III Organometallics 2024, 43, 1208–1212.
"Simple and Green Preparation of Tetraalkoxydiborons and Diboron Diolates from Tetrahydroxydiboron” Fornwald, R. M.; Yadav, A.; Montero Bastidas, J. R.; Smith, M. R., III; Maleczka, R. E., Jr. J. Org. Chem. 2024, 89, 6048–6052.
"Access to C(sp3) Borylated and Silylated Cyclic Molecules: Hydrogenation of Corresponding Arenes and Heteroarenes” Chhabra, A.; Reich, S.; Shannon, T. M.; Ghaffari, B.; Maleczka, R. E., Jr.; Smith, M. R., III RSC Advances 2024, 14, 10590–10607.
“Regiochemical Switching in Ir-Catalyzed C–H Borylation by Altering Ligand Loadings of N,B-Type Diboron Species” O’Connell, A. C.; Mansour, P. A.; Maleczka, R. E., Jr.; Smith, M. R., III Org. Lett. 2023, 25, 8057–8061.
“Steric Shielding Effects Induced by Intramolecular C–H•••O Hydrogen Bonding: Remote Borylation Directed by Bpin groups” Montero Bastidas, J. R.; Chhabra, A.; Feng, Y.; Oleskey, T. J.; Smith, M. R., III; Maleczka, R. E.; Jr. ACS Catal. 2022, 12, 2694–2705.
“Merging Iridium-Catalyzed C–H Borylations with Palladium-Catalyzed Cross-Couplings using Triorganoindium Reagents” Jayasundara, C. R. K.; Gil-Negrete, J.-M.; Montero Bastidas, J. R.; Chhabra, A.; Martínez, M. M.; Sestelo, J. P.; Smith, M. R., III; Maleczka, R. E.; Jr. J. Org. Chem. 2022, 87, 751–759.
“Amide Directed Iridium C(sp3)–H Borylation Catalysis with High N-Methyl Selectivity” Dannatt, J. E.; Yadav, A.; Smith, M. R., III; Maleczka, R. E.; Jr. Tetrahedron 2022, 109, 132578.
“One-Pot Iridium Catalyzed C–H Borylation/ Sonogashira Cross-Coupling: Access to Borylated Aryl Alkynes” Chotana, G. A.; Montero Bastidas, J. R.; Miller, S. L.; Smith, M. R., III; Maleczka, R. E., Jr. Molecules 2020, 25, 1754–1766.
“Para-Selective, Iridium-Catalyzed C–H Borylations of Sulfated Phenols, Benzyl Alcohols, and Anilines Directed by Ion-Pair Electrostatic Interactions” Montero Bastidas, J. R.; Oleskey, T. J.; Miller, S. L.; Smith, M. R., III; Maleczka, R. E., Jr. J. Am. Chem. Soc. 2019, 141, 15483–15487.
“C–H Borylation Catalysts that Distinguish Between Similarly Sized Substituents Like Fluorine and Hydrogen” Miller, S. L.; Chotana, G. A.; Fritz, J. A.; Chattopadhyay, B.; Maleczka, R. E., Jr.; Smith, M. R., III Org. Lett. 2019, 21, 6388–6392.
“Cobalt-Catalyzed C-H Borylation of Alkyl Arenes and Heteroarenes Including the First Selective Borylations of Secondary Benzylic C–H Bonds” Jayasundara, C. R. K.; Sabasovs, D.; Staples, R. J.; Oppenheimer, J.; Smith, M. R., III Maleczka, R. E., Jr. Organometallics 2018, 37, 1567–1574.
“Achieving High Ortho Selectivity in Aniline C-H Borylations by Modifying Boron Substituents” Smith, M. R., III; Bisht, R.; Haldar, C.; Pandey, G.; Dannatt, J. E.; Ghaffari, B.; Maleczka, R. E.; Jr.; Chattopadhyay, B. ACS Catal. 2018, 8, 6216–6223.
“Ir-Catalyzed ortho-Borylation of Phenols Directed by Substrate-Ligand Electrostatic
Interactions: A Combined Experimental / in Silico Strategy for Optimizing Weak Interactions”
Chattopadhyay, B.; Dannatt, J. E.; Andujar-De Sanctis, I. L.; Gore, K. A.; Maleczka,
R. E.; Jr.; Singleton, D. A.; Smith, M. R., III J. Am. Chem. Soc. 2017, 139, 7864–7871.
“Bismuth Acetate as a Catalysts for the Sequential Protodeboronation of Di- and Triborylated
Indoles” Shen, F.; Tyagarajan, S.; Perera, D.; Krska, S. W.; Maligres, P. E.; Smith,
M. R., III; Maleczka, R. E., Jr. Org. Lett. 2016, 18, 1554–1557.
“Reversible Borylene Formation from Ring-Opening of Pinacolborane and Other Intermediates Generated From 5-Coordinate Trisboryl Complexes: Implications for Catalytic C–H Borylation” Ghaffari, B.; Vanchura, B. A., II; Chotana, G. A.; Staples, R. J.; Holmes, D.; Maleczka, R. E., Jr.; Smith, M. R., III Organometallics 2015, 34, 4732–4740.
“Harnessing C–H Borylation/Deborylation for Selective Deuteration, Synthesis of Boronate Esters, and Late Stage Functionalization” Kallepalli, V. A.; Gore, K. A.; Shi, F.; Sanchez, L.; Chotana, G. A.; Miller, S. L.; Maleczka, R. E., Jr.; Smith, M. R., III J. Org. Chem. 2015, 80, 8341–8353.
“Silyl Phosphorus and Nitrogen Donor Chelates for Homogeneous Ortho Borylation Catalysis” Ghaffari, B.; Preshlock, S. M.; Plattner, D. L.; Staples, R. J.; Maligres, P. E.; Krska, S. W.; Maleczka, R. E.; Jr.; Smith, M. R., III J. Am. Chem. Soc. 2014, 136, 14345–14348.
“A Catalytic Borylation / Dehalogenation Route to ortho-Fluoro Arylboronates” Jayasundara, C. R. K.; Unold, J. M.; Smith, M. R., III; Maleczka, R. E.; Jr. Org. Lett. 2014, 16, 6072–6075.
“A Traceless Directing Group for C–H Borylation” PPreshlock, S. M.; Plattner, D. L.; Maligres, P. E.; Krska, S. W.; Maleczka, R. E.; Jr.; Smith, M. R., III Angew. Chem. Int. Ed. 2013, 42, 12915– 12919.
“High Throughput Optimization of Ir-Catalyzed C–H Borylation: A Tutorial for Practical Applications” Preshlock, S. M.; Ghaffari, B.; Maligres, P. E.; Krska, S. W.; Maleczka, Robert E.; Jr.; Smith, M. R., III J. Am. Chem. Soc. 2013, 135, 7572–7582.
“Outer-Sphere Direction in Iridium C–H Borylation” Roosen, P. C.; Kallepalli, V. A.; Chattopadhyay, B.; Maleczka, R. E., Jr.; Singleton, D. A; Smith, M. R., III J. Am. Chem. Soc. 2012, 134, 11350–11353.
“Practical One-Pot C–H Activation/Borylation/Oxidation: Preparation of 3-Bromo-5-Methylphenol on a Multi-Gram Scale” Norberg, A. M.; Smith, M. R., III; Maleczka, R. E., Jr. Synthesis 2011, 857–859.
“Electronic Effects in Iridium C–H Borylations: Insights From Unencumbered Substrates and Variation of Boryl Ligand Substituents” Vanchura, B. A., II; Preshlock, S. M.; Roosen, P. C. Kallepalli, V. A.; Staples, R. J.; Maleczka, R. E., Jr.; Singleton, D. A.; Smith, M. R., III Chem. Commun. 2010, 46, 7724–7726.
“Divergent Synthesis of 2,3,5-Substituted Thiophenes by C–H Activation/Borylation/Suzuki Coupling" Kallepalli, V. A.; Sanchez, L.; Li, H.; Gesmundo, N. J.; Turton, C. L.; Maleczka, R. E., Jr.; Smith, M. R., III Heterocycles 2010, 80, 1429–1448.
“Boc Groups as Protectors and Directors for Ir-Catalyzed C–H Borylation of Heterocycles” Kallepalli, V. A.; Shi, F.; Paul, S.; Onyeozili, E. N.; Maleczka, R. E., Jr.; Smith, M. R., III J. Org. Chem. 2009, 74, 9199–9201.
“Getting the Sterics Just Right: A Five-Coordinate Iridium Trisboryl Complex that Reacts with C—H Bonds at Room Temperature” Chotana, G. A.; Vanchura, B. A., II; Tse, M. K.; Staples, R. J.; Maleczka, R. E., Jr.; Smith, M. R., III Chem. Commun. 2009, 5731–5733.
“Iridium-Catalyzed Borylation of Thiophenes: Versatile, Synthetic Elaboration Founded on Selective C–H Functionalization” Chotana, G. A.; Kallepalli, V. A.; Maleczka, R. E., Jr.; Smith, M. R., III Tetrahedron 2008, 64, 6103–6114.
“Ir-Catalyzed Functionalization of 2-Substituted Indoles at the 7-Position: Nitrogen-Directed Aromatic Borylation” Paul, S.; Chotana, G. A.; Holmes, D.; Reichle, R. C.; Maleczka, R. E., Jr.; Smith, M. R., III J. Am. Chem. Soc. 2006, 128, 15552–15553.
“Aromatic Borylation/Amidation/Oxidation: A Rapid Route to 5-Substituted-3-amidophenols” Shi, F.; Smith, M. R., III; Maleczka, R. E., Jr. Org. Lett. 2006, 8, 1411–1414.
“One-Pot Borylation/Amination Reactions: Synthesis of Arylamine Boronate Esters from Halogenated Arenes” Holmes, D.; Chotana, G. A.; Maleczka, R. E., Jr.; Smith, M. R., III Org. Lett. 2006, 8, 1407–1410.
“C–H Activation/Borylation/Oxidation: A One-Pot Unified Route To Meta-Substituted Phenols Bearing Ortho/Para-Directing Groups” Maleczka, R. E., Jr.; Shi, F.; Holmes, D.; Smith, M. R., III J. Am. Chem. Soc. 2003, 125, 7792–7793.
“Remarkably Selective Iridium Catalysts for the Elaboration of Aromatic C–H Bonds” Cho, J.-Y.; Tse, M. K.; Holmes, D.; Maleczka, R. E., Jr.; Smith, M. R., III Science 2002, 295, 305–308.