Odom Research Group
 The Odom Research Group works at the interface between organic and inorganic chemistry with projects ranging
from drug discovery to understanding f-block metal reactivity. The focus is on advancing methodologies, especially those
related to Earth-abundant and ecologically-friendly titanium. There are several ongoing
projects in the lab. A brief description of some of the major projects is below.
The Odom Research Group works at the interface between organic and inorganic chemistry with projects ranging
from drug discovery to understanding f-block metal reactivity. The focus is on advancing methodologies, especially those
related to Earth-abundant and ecologically-friendly titanium. There are several ongoing
projects in the lab. A brief description of some of the major projects is below.
- With the Liby Research Group in the Pharmacology & Toxicology Dept we are developing NRF2 inhibitors, a project that involves several new organic methodologies for pyridine synthesis including early and late transition metal catalysis. The project aims to make chemotherapeutics more effective for difficult to treat cancer lines. We collaborate with the Gallo Research Group in Physiology as well, and they have shown that NRF2 inhibitors produced in our laboratory decrease cancer cell mobility and metastasis.
- Methods for high oxidation state catalyst development are not as well established as their low oxidation state counterparts. Our research group has developed a method for determining the donor ability of common anionic ligands for high oxidation state metals, e.g., halides, alkoxides, aryloxides, amides, pyrrolides, etc. The new descriptor is simply called the Ligand Donor Parameter (LDP), which can be used in conjunction with other descriptors to model high oxidation state catalysis.
- We are interested in the preparation, properties, and reactivity of novel f-block systems. For example, we reported a rare example of a U(II) complex in collaboration with the Boncella group, then at Los Alamos National Lab now at Washington State. Currently, we are examining unusual Y(II) and lanthanide(II) complex. The magnetic behavior of the complexes is being studied in collaboration with the Demir Group. Further, we are interested in preparing novel catalytic complexes of the f-block to see if they can be modelled using LDP.
For these, we often employ a variety of different synthetic methodologies, quantum chemical methods (especially DFT and NBO), and reaction modeling to understand and advance the project.

Organic Methodology and Drug Discovery
We have developed new titanium-catalyzed and -mediated reactions in heterocyclic synthesis for decades in our research group. Chief among these is a titanium-catalyzed multicomponent coupling reaction, iminoamination. The reaction involves the 3-component coupling between an alkyne, isonitrile, and primary amine to generate derivatives of 1,3-diimines. This process has been extensively used in one-pot syntheses of heterocycles. The types of syntheses developed are shown in the exploding diagram below.
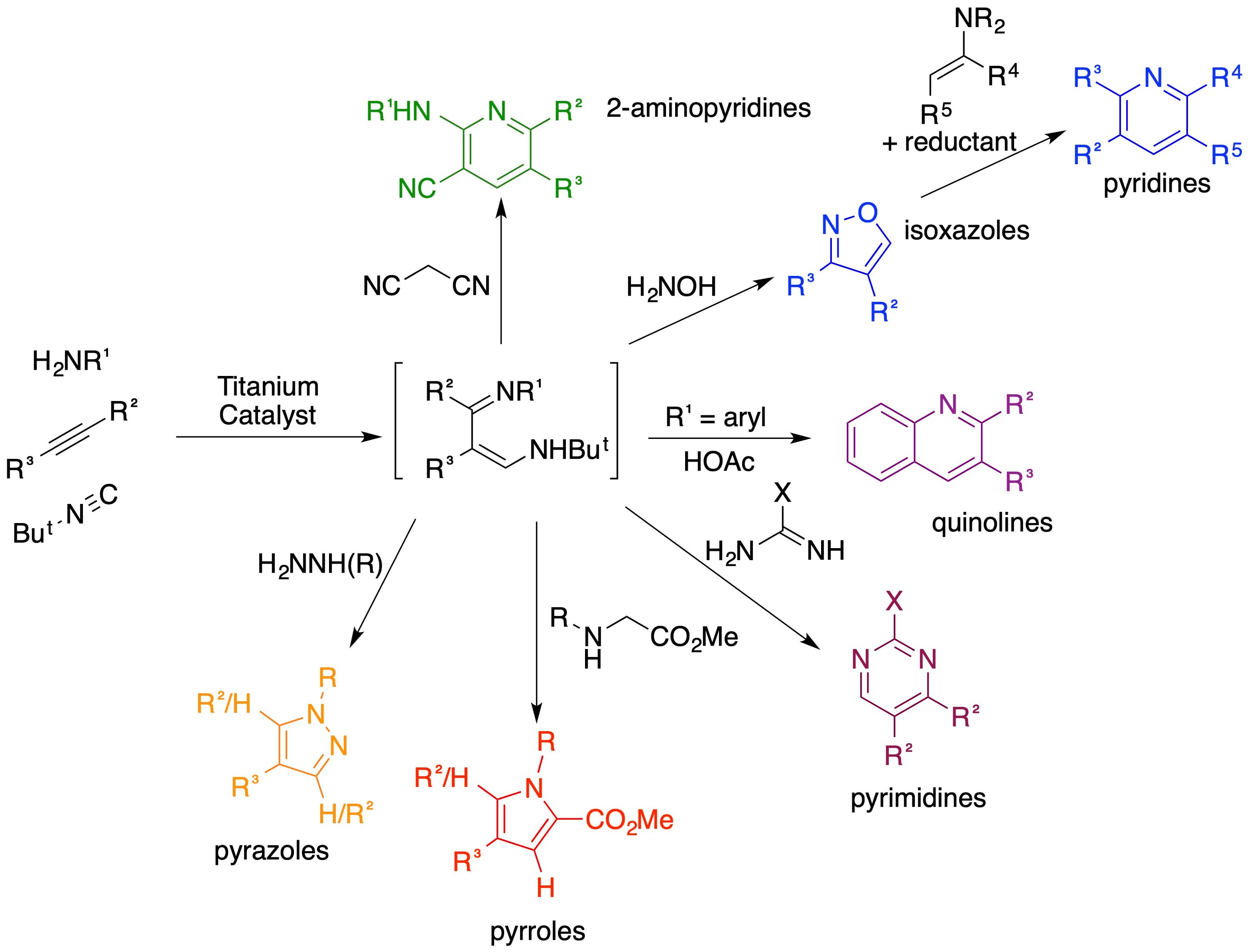
Of particular note is the synthesis of 2-amino-3-cyanopyridines via a one-pot procedure. It was discovered that certain members of this class of compounds are NRF2 inhibitors by the Liby Research Group. The NRF2 signaling pathway is responsible to cellular response to a variety of different stressors such as oxidants and carbocations. NRF2 is typically associated with its inhibitor KEAP1 in our cells. In the presence of many molecular assailants, such as oxygen radicals or sulfuraphane from cruciferous vegetables, KEAP1 releases NRF2, which travels to the nucleus to turn on MAF-dependent transcription in the Antioxidant Response Element (ARE). The ARE codes for over 100 different proteins including superoxide dismutase, catalase, and ABC efflux proteins.
Ordinarily, activation of the NRF2 pathway is an encouragement to good health, i.e.,
we are encouraged to eat broccoli; however, some cancers including 30% of lung cancers
will overexpress NRF2 making the cancer cells resistant to chemotherapeutics and radiotherapy.
Combination of an NRF2 inhibitor with the chemotherapeutic can make treatment more
effective. For example, Liby and coworkers examined xenographed human lung cancer
cells with a constitutively activated NRF2 pathway in mice (plot below). The blue
and red lines show tumor volume vs days when given vehicle and a low dose of carboplatin,
a commonly employed anticancer drug developed here at MSU. As you can see, there is
no significant effect of carboplatin treatment on tumor volume for these NRF2-activated
cells. On treatmen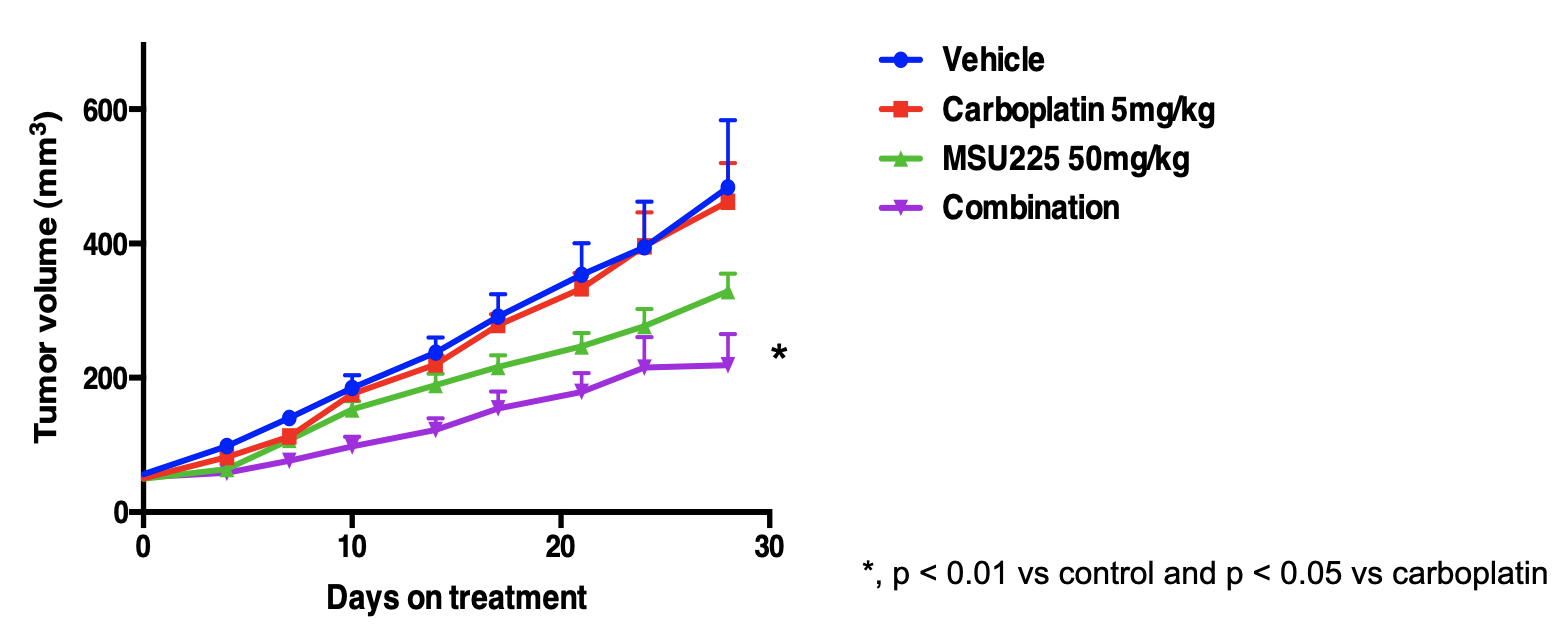 t with MSU225, a compound prepared using our titanium catalysis, tumor growth is inhibited
(green line), but the combination of low dose carboplatin and MSU225 dramatically
slows tumor growth.
t with MSU225, a compound prepared using our titanium catalysis, tumor growth is inhibited
(green line), but the combination of low dose carboplatin and MSU225 dramatically
slows tumor growth.
The project has used titanium, copper, and palladium catalysis to generate over 100 derivatives of MSU225. In the process, we have discovered more potent and drug-like compounds that are currently under study. In addition, we are developing new methodologies for the the synthesis of pyridines.
Some leading references on this project:
“A Novel NRF2 Pathway Inhibitor Sensitizes KEAP1-mutant Lung Cancer Cells to Chemotherapy,” Zhang, Di; Hou, Z.; Aldrich, K.; Lockwood, L.; Odom, A. L.; Liby, K. T. Molecular Cancer Therapeutics, 2021, 20 (9), 1692-1701. https://doi.org/10.1158/1535-7163.MCT-21-0210
“Titanium-Catalyzed Multicomponent Couplings: Efficient One-Pot Syntheses of Nitrogen Heterocycles,” Odom, A. L.; McDaniel, T. J. Acc. Chem. Res. 2015, 48, 2822-2833. https://doi.org/10.1021/acs.accounts.5b00280
New Tools for Catalyst Development and Reaction Modeling
 In this project, we are examining the use of high oxidation state complexes for parameterizing
ligand donor ability in d0 metal complexes. There are many well-known reactions where the donor ability of ancillary
ligand sets affect the overall catalytic activity of a compound. However, it can be
difficult to evaluate the donor abilities of structurally diverse ligands, e.g., pyrrolyl
versus phenoxides. Here, we are trying to experimentally identify the donor ability
of ligands and correlate that donor ability to spectroscopic measurements and reactivity
in a diverse array of early transition metal systems. For this, we have developed
a chromium(VI) nitride system, NCr(N-i-Pr2)2X, where X = the ligand under interrogation. Because there is significant Cr-NPr2 π-bonding and X competes with the same acceptor orbitals on the metal, increased
donation from X leads to less Cr-amide π-bonding. The rate of amide rotation can be
measure by 1H NMR using spin-saturation transfer, and the rate is related to our Ligand Donor
Parameter (LDP). The LDP of over 100 different ligand types have been measured, and
this descriptor can be used to model high oxidation state catalysts, allowing prediction
of reaction rate based on structure in some cases.
In this project, we are examining the use of high oxidation state complexes for parameterizing
ligand donor ability in d0 metal complexes. There are many well-known reactions where the donor ability of ancillary
ligand sets affect the overall catalytic activity of a compound. However, it can be
difficult to evaluate the donor abilities of structurally diverse ligands, e.g., pyrrolyl
versus phenoxides. Here, we are trying to experimentally identify the donor ability
of ligands and correlate that donor ability to spectroscopic measurements and reactivity
in a diverse array of early transition metal systems. For this, we have developed
a chromium(VI) nitride system, NCr(N-i-Pr2)2X, where X = the ligand under interrogation. Because there is significant Cr-NPr2 π-bonding and X competes with the same acceptor orbitals on the metal, increased
donation from X leads to less Cr-amide π-bonding. The rate of amide rotation can be
measure by 1H NMR using spin-saturation transfer, and the rate is related to our Ligand Donor
Parameter (LDP). The LDP of over 100 different ligand types have been measured, and
this descriptor can be used to model high oxidation state catalysts, allowing prediction
of reaction rate based on structure in some cases.
For example, titanium-catalyzed hydroamination has been studied for over 2 decades,
and it has been generally shown that more electron-deficient ligand sets are better
for the reaction. However, this brings up a difficult question, what is an electron-deficient
ligand set for a high oxidation state metal center? In contrast to low oxidation state
system, e.g., Pd(II), where many of the metal-based orbitals are occupied by unpaired
electrons, high oxidation state metals are typically primed to act as π-acceptors
to lone pairs on electronegative atoms like nitrogen and oxygen. Consequently, pKa
is a poor descriptor for donor ability. Using the LDP descriptor, we can determine,
for example, that tert-buto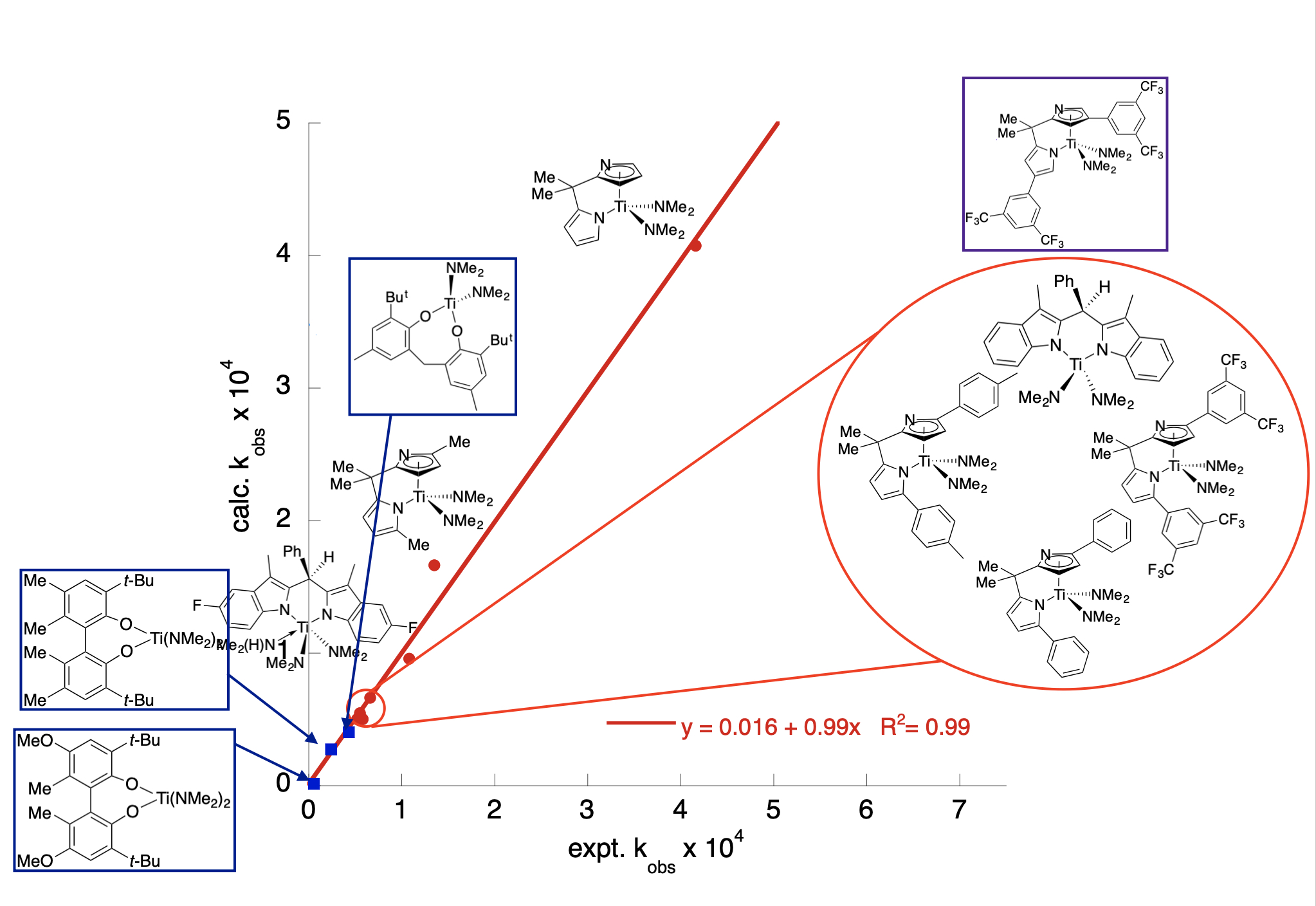 xide > F- > pyrrolide > Cl- > triflate in donor ability. Further, we can quantify the donor ability use that
to fit kinetic data for a reaction. In the plot below, we examine symmetrical, bidentate
ligands and their effect on catalysis and developed a quantitative model, kobs ∙ 104 = −6.88 + 1.75(LDP) − 0.6345(%Vbur), where %Vbur = percent buried volume (a steric descriptor). Plugging in the LDP and %Vbur values for a new symmetrical bidentate ligand gives the rate of the reaction as long
as no mechanism changes occur.
xide > F- > pyrrolide > Cl- > triflate in donor ability. Further, we can quantify the donor ability use that
to fit kinetic data for a reaction. In the plot below, we examine symmetrical, bidentate
ligands and their effect on catalysis and developed a quantitative model, kobs ∙ 104 = −6.88 + 1.75(LDP) − 0.6345(%Vbur), where %Vbur = percent buried volume (a steric descriptor). Plugging in the LDP and %Vbur values for a new symmetrical bidentate ligand gives the rate of the reaction as long
as no mechanism changes occur.
Currently, we are expanding this modeling process to more complex systems, which is allowing us to discover new effects in related catalyses that might have been difficult to identify without this procedure.
Some leading references on this project:
“Quantifying Ligand Effects in High Oxidation State Metal Catalysis,” Billow, B. S.; McDaniel, T. J.; Odom, A. L., Nature Chemistry 2017,9, 837-842. http://doi.org/10.1038/NCHEM.2843
“Catalyst Design Insights from Modeling a Titanium-Catalyzed Multicomponent Reaction,” Aldrich, K. E.; Odom, A. L. Faraday Discussions, 2019, 220, 208-230. http://doi.org/10.1039/c9fd00033j
“Evaluation of Donor and Steric Properties of Anionic Ligands on d0-Metals”, DiFranco, S. A.; Maciulis, N. A.; Staples, R. J.; Batrice, R. J.; Odom, A. L. Inorg. Chem. 2012, 51, 1187-1200. http://doi.org/10.1021/ic202524r
Advances in f-Element Chemistry
%20Synth.jpeg) The f-block of elements constitute a burgeoning frontier of chemical reactivity where
ligand donor abilities and sterics are balanced with covalent and ionic bonding effects.
In one published work, we examined the synthesis and properties of a U(II) complex
in collaboration with Jim Boncella and coworkers at LANL. A triarylamide ligand (NHAr)
acts as a chelate towards uranium with one of the aromatic rings in each amide acting
as an η6-donor in U(NHAr)2. At the time, there were only two other examples of uranium(II) molecular species
reported, and they had different electronic configurations: the expected f4 and surprising d1f3. In this example, the expected f4 electronic configuration is obtained. This was supported with EPR data in collaboration
with Prof. McCracken at MSU.
The f-block of elements constitute a burgeoning frontier of chemical reactivity where
ligand donor abilities and sterics are balanced with covalent and ionic bonding effects.
In one published work, we examined the synthesis and properties of a U(II) complex
in collaboration with Jim Boncella and coworkers at LANL. A triarylamide ligand (NHAr)
acts as a chelate towards uranium with one of the aromatic rings in each amide acting
as an η6-donor in U(NHAr)2. At the time, there were only two other examples of uranium(II) molecular species
reported, and they had different electronic configurations: the expected f4 and surprising d1f3. In this example, the expected f4 electronic configuration is obtained. This was supported with EPR data in collaboration
with Prof. McCracken at MSU.
Currently, we are examining other M(NHAr)2 species, which show unusual reactivity and properties. The magnetic behavior of Ln(NHAr)2 complexes is being examined in collaboration with the Demir Group at MSU. In other projects, we are using LDP-based modeling to understand actinide and lanthanide reactivity, properties, and catalysis.
magnetic behavior of Ln(NHAr)2 complexes is being examined in collaboration with the Demir Group at MSU. In other projects, we are using LDP-based modeling to understand actinide and lanthanide reactivity, properties, and catalysis.
Some leading references on this project:
“Synthesis and Characterization of a Neutral U(II) Arene Sandwich Complex,” Billow, B. S.; Livesay, B. N.; Mokhtarzadeh, C. C.; McCracken, J.; Shores, M. P.; Boncella, J. M.; Odom, A. L., J. Am. Chem. Soc., 2018, 140, 17369-17373. http://doi.org/10.1021/jacs.8b10888
"A rare isocyanide derived from an unprecendented neutral yttrium(II) bis(amide) complex." Jena, R.; Benner, F.; Delano IV, F., Holmes, D.; McCracken, J.*; Demir, S.*; Odom, A. L.*Chem. Sci. 2023, 14, 4257–4264. https://doi.org/10.1039/D3SC00171G
